J.ophthalmol.(Ukraine).2017;2:51-69.
|
https://doi.org/10.31288/oftalmolzh201725169 Adaptive myopia
Part 3. Interaction of physiological mechanisms of eye’s focusing with mechanisms of acquired myopia development 1Koshits I.N. 2Svetlova O.V. 1Petercom-Networks / Management Systems Consulting Group Cl. corp., St. Petersburg, Russia; 2The I.I. Mechnikov Northwestern State Medical University, St. Petersburg, Russia.
We reviewed morphology and physiology of the macular area of the eye and performed the analysis, adequate to the optics laws, of an input optic signal coming to the fovea from white light rays dispersed (into the spectrum) at the inner corneal surface: these are not light scattering circles but excitation bands of red and yellow cones in the fovea and of blue cones in surrounded periphery. We analyzed in details a hypothesis on the presence of a functional mechanism of “comparing the position and intensity of excitation planes of red, green, and blue cones” in the fovea. The work of the operating mechanisms of eye’s focusing found was reviewed in regard to a metabolic theory of acquired myopia which we had proposed earlier (2006). It was found that acquired myopia is a classic case of absolute prevalence of accommodation control system over aqueous humor outflow control system when their common mechanism, the ciliary muscle, first of all, executes the accommodation function. An operating mechanism of adaptive elongation of the axial length, which was found by authors, is common for human beings and animals. So, initial myopia is not a disease but a normal adaptation of the eye to a certain visual life environment. This, in fact, is a direct action of life efficiency law in biological system development since the eye after adaptive LA elongation can perform stressful visual work with less tension of the ciliary muscle, i. e. with decreased blood supply. Comparative clinical observation was performed by M.G. Guseva during 3.5 and 7 years. Outcomes obtained from 2 492 patients using all modern means of spectacle and contact correction first confirmed the rationale of the metabolic theory of myopia development by uploading and loading type. Practical recommendations were given for effective prevention and control of acquired myopia with early rational optical correction taking into account individual visual acuity of a patient. Till there are no visual work regulations, the issues of videoecology and total prevention of blindness take the central stage in fight against the most massive “myopia pandemic” in the history of mankind. Key words: adaptation, acquired myopia, metabolic theory, early rational correction, visual acuity, video ecology, visual work regulations, bands of excitation in the macula, systems to control accommodation, length, and focusing of the eye
In our previous papers[8], we have drawn attention to the fact that this is not “sharpness of the image” but pre-assessment of inter-position of red, green and blue bands (RGB-bands) of excitation in the macular is a qualitative and highly-sensitive input signal for the whole accommodation control system functioning. The issue of how a dispersive mechanism of eye’s focusing works in the myopic eye will be described in the present paper. 3. A dispersive mechanism of eye’s focusing What kind of “geometry” of excitation bands should the macular expect? Are these light scattering CIRCLES or excitation BANDS? Although this issue is between two disciplines, optics and physiology, it, without doubts, requires a word of clarification. Let us recollect a classic experiment performed by Isaac Newton in his place in Woolsthorpe in 1966. Newton made a small round hole in the window shutter, through which a light beam got into the dark room; and on its way, Newton placed a glass triangular prism, in which a white light ray was dispersed. On the opposite wall at the distance of 6 meters appeared a multicoloured band which Newton called spectrum (Latin spectrum means "image" or "apparition"). The phenomenon itself was called dispersion (Latin dispersio means “scattering”) (Fig.1).
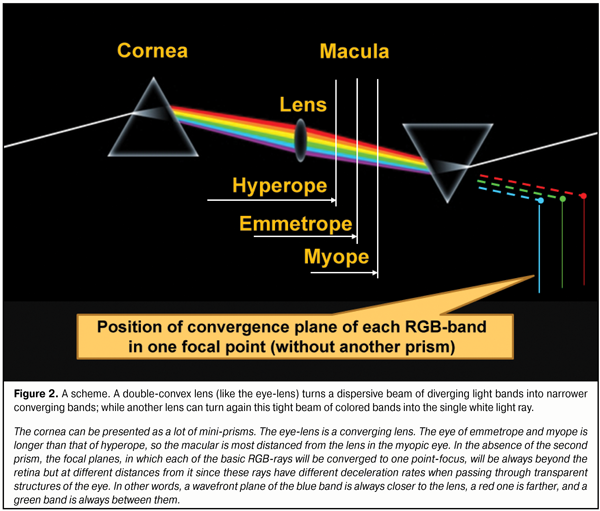
His experimental series showed that the white light consists of different color rays and the angle of refraction of each ray is different and depends on “its physical entity”. Newton explained this phenomenon by differences in masses of corpuscles. It is amazing but Isaac Newton, perhaps, did not draw his attention to another remarkable phenomenon: when passing through the round window shutter and, then, through the prism, a “round” white light, dispersed into colors, created on the wall not light scattering circles but a clear linear spectrum. It is possible to try to explain this fact by a various effect of gravitation on the light. But the cornea and the lens, in point of fact, also consist of many mini-prisms! And the white light, upon passing through the cornea with an index of refraction (IR) of 1.376 into the aqueous humor with IF=1.336 [48], has, likely, to undergo the dispersion in passing from a stronger optic medium to a weaker one. Some investigators have also noticed a light scattering on the inner surface of the cornea or in the eye fundus [2]. And, of course, the eyes of human beings and of a number of animals must be so structured that the macula received not light scattering circles but the linear bands of the red, green and blue rays (RGB-bands). This key statement determines, in fact, the physiology of our vision: for in the general spectrum of the input optic stimulus there must be highlighted the three-colored linear bands, the color and borders of which can be detected by the macula with three types of cones identifying only blue, green, and red colors. It should be noted that the cornea is like a concavo-convex scattering lens that diffracts each RGB-band, appearing after the white light dispersion, outwards the optic axis. So, these RGB-bands come from the cornea to the lens as diverging bands and they become converging as they are refracted when passing through the lens structures. It is also important to understand that, when passing through the lens optic structures, the width of the basic RGB-bands coming from the cornea will remain unchanged; but, on the anterior surface of the lens, the angle deviation of each band at different accommodation phases will be relatively different depending on the band color, i.e. on the degree of its propensity for deviation. Besides, upon passing through the converging lens, the eye-lens, the width of excitation bands from RGB-rays will decrease as they converge to the macular. This principle of reversibility has been checked in many experiments and it enables re-converge all the light striking bands from all spectral rays into the white light ray by means of the double-convex lens and the prism as it is shown in Fig. 2.
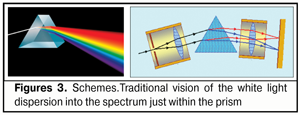
To speak in images, in fact, a kind of “traffic lights-projector” with three basic RGB-bands is formed on the posterior surface of the lens. Herewith, the width and the vertical partitioning area of these bands on the posterior wall of the lens capsule will be determined by the lens thickness, radius of its both surfaces as well as by its current position on the optic axis at different accommodation phases. The cornea can be presented as a lot of mini-prisms. The eye-lens is a converging lens. The eye of emmetrope and myope is longer than that of hyperope, so the macular is most distanced from the lens in the myopic eye. In the absence of the second prism, the focal planes, in which each of the basic RGB-rays will be converged to one point-focus, will be always beyond the retina but at different distances from it since these rays have different deceleration rates when passing through transparent structures of the eye. In other words, a wavefront plane of the blue band is always closer to the lens, a red one is farther, and a green band is always between them. To summarize, we can say that in accommodation, the eye-lens refract not RGB-rays but RGB-bands, the deviation level of which will depend on simultaneous operation of three lens biomechanisms: changes of refracting power of the anterior and posterior lens surfaces displacement of the lens along the optic axis increase of the lens thickness that leads to the additional divergence of RGD wavefronts along the axial length because of changes in braking path rates in the lens masses. Today, it has become clear that dispersion (“diffusion”) of the light is a result of interaction of the optic (electromagnetic) wave with those charged particles which enter into the composition of the refractive medium. Today, dispersion is called the dependence of refractive index n of a medium on frequency ? (wave length ?) of a light. It should be noted that phase spread velocity in vacuum is equal for any color optic wave and is equal to the velocity of light, с = 3 х 108 m/s. The color is determined by the light wave frequency and its spread velocity depends only on the refractive index of the optic medium. But where does dispersion take place? Is it only on the border of the media or inside the media? Although Newton discovered light dispersion, he did not explain completely its essence; though he believed back then that “light corpuscles hitting the medium cause the waves in it” [23]. This takes nothing away from greatness of Newton: it is easy for us to argue today after the wave and corpuscular light theory has been discovered! However, there are special features of the white light’s passing through the transparent prism which have to be discussed since they affect the form and position of the input signal from the RGB-bands in the macula: 1. Whether the white light is dispersed into the circles or BANDS of light scattering?! 2. Whether the light is spread out into a spectrum on the first prism face or only on the second face of the prism? 3. Whether the light dispersion occurs within the prism or only on its borders when contacting to a less dense optic medium? Understanding these specific features will give us clear vision of the structure and parameters of the input optic signal, coming in the macula, as well as, for instance, which of surfaces of the optic lens, cornea, or intraocular lens, anterior or posterior, in fact, executes the splitting of the white light into the components. Traditionally, the white color, when passing through the prism, is split into the spectrum once entered the optic media of the prism (Fig. 3).
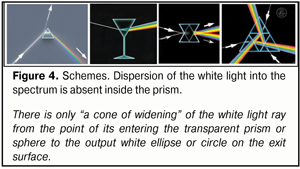
Newton, probably, thought the same way when he performed a successful experiment in which, using a narrow slot, only green light rays were extracted from the spectrum at the posterior surface of the prism. However, practically, we do not observe “rainbow” glowing inside the prism or inside a gem when the white light is passing through them. This “rainbow” is seen only on their surfaces (Fig. 4, 5).
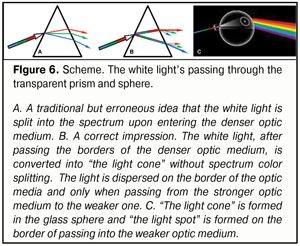
If the light split into the spectrum as early as inside the prism, a thin semi-transparent sheet of paper placed on the exit surface of the prism or, for instance, a glass sphere, would reflect a “rainbow” formed inside. But we do not see that; we can see only an output light spot (Fig. 6).
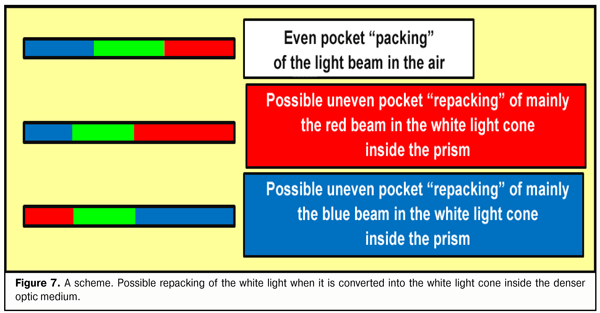
This means that no dispersion occurs inside the prism, there is only “spreading” out the linear synergetic white light ray into “the cone of light”. Appearance of the light cone unsplit into the components, without doubts, is associated with different energetic superposition strength of frequencies comprising its spectrum. The light is a kind of “getting prepared” to be split when exiting from the stronger optic medium into the weaker one; the light is gathered inside the light cone in unslit, so far, beams, in which the most powerful are the components of conditionally violet-blue, green, and red lights that have transferring energy close to each other. Likely, such a preliminary filtration package “repacking” (in other words, changing the superposition of the frequencies) of RGB-rays within the white ray inside the light cone eases its dispersion into the spectrum when passing from the “stronger” to “weaker” optic medium (Fig. 7).
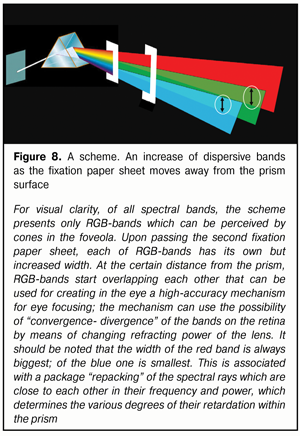
As a rule, we strongly believe that if we put a white semi-transparent sheet of paper to the posterior “exit” surface of the prism, we must see there, as Newton saw on the remote screen, a rainbow band, though a narrower one. However, we will not see it. But if we take this paper sheet a bit farther, we will see not circles (!) but light scattering bands; and the farther from the prism the paper sheet is the bigger vertical size have these bands (Fig. 8).
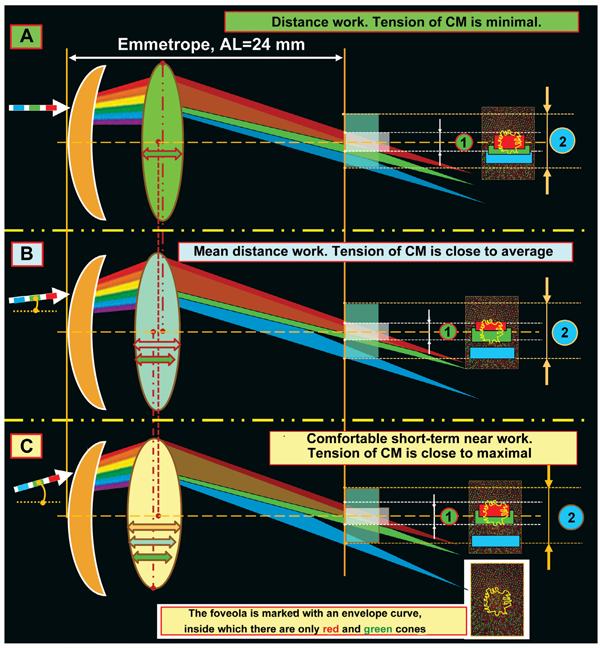
Also, if we move the paper sheet up and down along the exit prism surface, the color saturation on the different color light scattering bands will be decreased: this is so because we, actually, cover with “a stove-lid” a part of energy from rays, which are split so far into the spectrum. However, in our opinion, the most important thing is that geometric sizes of these light scattering bands will always vary in proportion to each other when the distance between the prism and the paper sheet is different! Everyone can create this “real miracle” in a simple experiment at his own place as a Newton’s assistant. And, being a convergent lens, the eye-lens will also be able to change the degree of convergence- divergence of RGB-bands at various accommodation phases; and this fourth biomechanism of the lens, from our point of view, seems to be most important for highly-effective functioning of eye’s focusing accommodative mechanism. These ideas are illustrated in Figure 9.
Figure 9 demonstrates facts as follows. The ring around the foveola has a high level of blue cone concentration and is, likely, a kind of “sighting unit” when the eye’s optic center is initially fixated on an object sighted and, afterwards, a physiological mechanism of accurate eye’s focusing is put into operations by means of accommodation ability of the lens to converge-diverge as well as to overlap GB-bands at different accommodation phases. It should be noted that the ciliary muscle is reflective since it has a smooth morphological structure; so it is slower as compared with any striated muscle. But when “an eye sighting unit”, made of a blue cone ring, focuses on a possibly dangerous object, ciliary muscle activation is not required and, most probably, all it takes are saccadic binocular movements of both eyes. I.e. it appears that, at first, we visually and binocularly fix the area of “sighting” with the blue cone ring around the foveola and, only after that, we turn on accommodation if necessary. And, in fact, this is a highly effective binocular focusing system with “a telescope-sight”! Besides, natural regular astigmatism which remains in all human beings just almost to old age helps “collect” in the vertical meridian a clearer blue band in the ring around the foveola due to the greater concentration of optic rays coming from the top and bottom of the object appeared. This physiological mechanism gives us the possibility to assess immediately vertical sizes of the danger, i.e. we can partially control axial chromatic aberration in a vertical plane, which, of course, is important for survival of species. Therefore, attention should be paid to the fact that some optometrists’ ideas on complete leveling of natural physiological vertical astigmatism are, likely, ill-founded. Figure 9 also demonstrates that the emmetropic (like hyperopic) eye is made suitable for comfort and secure distance work in the that make it possible to recognize quickly and clearly the high-treat object by receiving a rather powerful signal from each of RG excitation band overlapped on each other. We should note that the hyperopic eye can better perform this operation than the emmetropic eye since the degree of RG- excitation band overlapping in the foveola will be maximal. When the emmetropic eye focuses in distance work (Fig. 9A), the signal from the red excitation band activates a much greater number of red cones in the foveola as compared to a number of green cones activated. This will guarantee a high-quality output electric signal from RG excitation bands in the fovea to the brain.
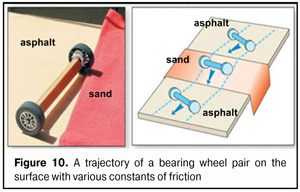
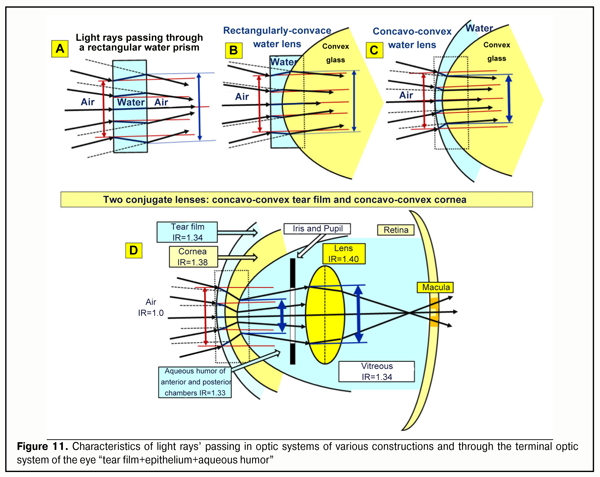
We know very well that to improve far focusing, long-distance drivers and large display operators successfully use yellow-color filters. The success of using yellow-color filters, which are closer to the red part of the spectrum than to the green one, is, likely, associated with an increase in image contrast since there is an adequate “decrease in power” of the green excitation band in the fovea (Fig. 9A) and a greater area of the red cones, spectrum of which includes also a yellow component, is involved. And if that is so, then the physiologic mechanism of a red-green test which is used by optometrists to select corrective lenses for far distance becomes clearer: the corrective lenses are considered to be properly chosen when a visual stimulus is equally sharp when seeing both with the red and green filters. And this is possible only in case of the equal light striking areas of GR excitation planes in the foveola. The equality of light striking areas in the foveola is observed in eye’s working for mean distance (Fig. 9B). In brief, the red-green test adjusts the eye optics for mean distance. Besides, visual near work (Fig. 9B) can be more comfortable in such artificial light the spectrum of which is closer to the red spectral area. And in this case, a summed signal of excitation in the foveola will partially be retargeted for additional activation of the narrower red cone excitation plane. More likely, that is why, for comfortable visual near work, we should increase not only illumination level but also use lamps with a spectrum close to the sun spectrum and with corresponding organization of the red spectral area to which the retina is most sensitive [5]. Everyone can learn at first hand the high comfort when reading in the light of a “warm” yellow lamp with the close-to-sun spectrum, also in regards to light temperature. And this is an important conclusion for hygienists who address the issues of video-safety of artificial light sources. The foveola has been found to contain only red and green cones and the sensitivity of the green cones is many times higher than of the red ones. It is logical to assume that the green cones, first of all, detect the quality of the input optic signal and this is the green excitation band that fine sharp focusing is, likely, associated with. In the ring around the foveola, there are only blue cones the sensitivity of which is 100 times higher than that of the green cones. This ring reminds “a telescope-sight” and its first task is to extract a recognizing object from the general background picture and “to catch” it inside the foveola as an object-target to be analyzed in details. Apparently, only in this case, the brain can go on finer focusing. It should be noted that in the nature and in the technology, the most sensitive sensors are those based on relative movement of any structure in the space being measured. All mentioned above means that the very physiological possibility of clear vision, most probably, is defined by sizes and axial-length-related transversal run of the green excitation band at all those moments when, simultaneously, the blue cones are also activated in the peripheral ring around the foveola. If there is no blue ring activation around the foveola, the brain, likely, will not be able to make a control signal to maintain the position and the shape of the lens; and the signal can provide transition to finer eye’s focusing trough regulating relative positions of GR excitation planes within the foveola area. Analysis of Figure 9 can make physiological conclusions as follows: 1. The input optic signal on the focal plane in the foveola is characterized not only by the area of “light striking” of the excitation bands from RGB-rays but also by their geometric position to each other. 2. The physiological mechanism of overlapping-converging-diverging of the excitation bands on the focal plane in the foveola can define the moment of optically clear eye’s focusing on the focal plane by the exact moment when the red and green excitation bands are converged (exact contacting or overlapping) in the presence of initial compulsory appearance of the blue cone excitation plane in the optic sighting unit of the eye. 3. An individual width of RGB excitation bands on the focal plane in the foveola varies at all accommodation phases from minimal when looking far in the distance to maximal when looking near. 4. Changes in CM tension lead to response changes of lens refracting power and can directly change inter-relative positions of excitation bands on the focal plane in the foveola. 5. The capacity of human visual system to realize the whole accommodation functioning is, likely, that individual refraction in each eye when looking far in to the distance can provide partial overlapping of the green and red excitation bands on the focal plane in the foveola. 6. To provide high-quality binocular vision in both eyes, for instance, in visual mean distance work, such simultaneous conditions are, likely, required: 1) achievement of the equal degree of convergence of the green and red excitation bands on the fixation plane in the foveola and 2) simultaneous excitation of the blue cones in the optic sighting unit of each eye. These theoretical conclusions, in some measure, can explain unclear so far clinical facts which have been obtained from studying visual work of the eye using computer accommodography [6]. Let us note a few important moments more. We are in the habit of thinking that the light rays coming into the eye are refracted only by the cornea. However, this is not exactly so. At first, the incoming light rays pass through the first “liquid” lens: the tear film with the epithelium that has a certain thickness. And, only then, it goes through the second lens which is the cornea. There is no air between these two lenses, and they are, in fact, in tight contact. The figure 10 helps to understand a scheme, which is adequate to geometrical optic laws, for the rays’ passing through the various optic systems (A, B, C) that are close to the optic system (D) in regard of their “biological construction: “tear film+epithelium+cornea” (Figure 11).
Figer 10 demonstrate a classic school example that explains which way the light rays have to turn when they pass angle-wise from the media with a weak index of refraction (asphalt in the figure) into the stronger optic medium (sand in the figure). The light ray is shown as a bearing pair. This scheme can avoid mistaking when designing a geometrical way of the rays in any optical system. When one bearing wheel runs onto the puddled sand, it gets slower (the force of friction is increased). Herewith, the second wheel still runs a certain distance on the asphalt, where the force of friction is low, with an initially higher speed. This will lead, at first, to a turn of the main axle of the wheelpair and, then, to its further straight movement on the puddled sand with a lowered speed. When leaving the sand, there will be a reverse process; and a trajectory of the wheelpair (or a light ray) will be a bit off-centered but parallel to the trajectory before the entrance onto the sand. This case corresponds to Figure 11A when the light rays passes through a rectangular water lens. The attention should be paid to the fact that a diameter of the incoming ray cone light striking increases upon passing through such a lens (the “output” diameter of the light striking is larger than the input one, blue arrow). Ant this phenomenon can be clearly observed when the light goes through thick glasses of sky-scrapers or through thick bullet-resistant glasses of armored vehicles.
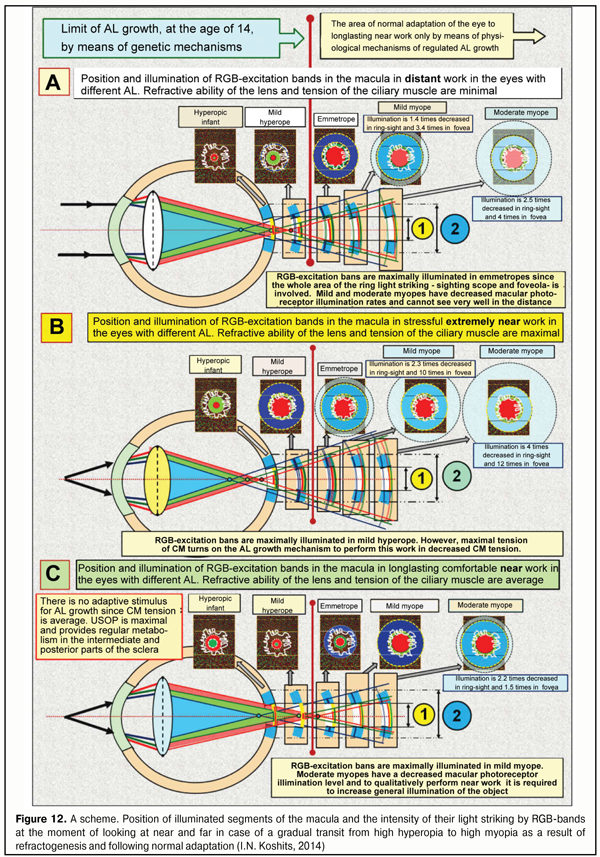
This is associated with the fact that the index of refraction is significantly higher in water than in air, IR=1.33/1.00, respectively; as well as with another fact that input and output optic surfaces of the rectangular water plate are parallel to each other but not at an angle like, for instance, in the prism. The light striking diameter directly depends on the thickness of the water prism: the thicker such a prism, the bigger the diameter of light striking, i.e. the bigger the degree of divergence of the optic rays. The influence of the thickness and topography of the tear film with the corneal epithelium on the general refractability of the eye is noticeable enough. Night-wear orthokeratological contact lenses, first of all, change the spatial geometry, the thickness, and the optic refractive power of the epithelium, and, therefore, the optic power of the tear film, which is connected to the epithelium, since the epithelium thickness is increased along the edges and decreased in the center; thus, the tear film with the epithelium turns temporarily into s stronger dispersive lens that is temporarily able to inflect rays more from the optic axis of the eye. Herewith, the eye has a temporary hyperopic refraction, which enables to successfully retard or to effectively stop mild or moderate acquired myopia. It is also important to note that orthokeratology changes no geometry of the cornea itself but only changes spatial geometry and, respectively, refractability of the corneal epithelium and the tear film. That is why orthokeratology can be considered not only a reversible but sparing effect. All mentioned above mean that any reversible change in the corneal topography after refractive surgery will lead to local changes in the corneal epithelium and tear film thickness and, in consequence, to noticeable changes of its refractive properties both in regard to the intensity of “pressing” of a ray pass line to the optic axis and in regard to the travel direction of the rays coming out of it to the cornea. This important circumstance is not always taken into account when planning refractive surgery or, for instance, in diagnostics and treatment of keratoconus when spatial geometry of the epithelium and the tear film are partially changed due to alteration occurred in the topography of the cornea itself. If the optic system is designed as “plano-concave water lens + convex glass lens” so a decreased turn of the light beam after their entrance into the optically denser glass will be observed (Figure 11B). This is so because, like in our example from school, one of the pair wheels will pass so far a larger distance on “the pressed sand” of the water rectangular lens as opposed to “the loose sand” of the convex glass lens. Although the light striking ring diameter will decrease more because “the water lens” will be thinner in the center due to the glass lens laid over, the rays will become less convergent so far. And what if the plane water lens will become convex-concave? This case is given in Figure 11 C where a convex-concave lens is in a tight contact with a convex glass lens. It is well seen that, in this case, the degree of a relative pressing of the optic axes of the rays to the general system optic axis will be increased, i.e. the general light striking diameter will be some more decreased (see blue arrows) and, moreover, these rays will become almost parallel to the general optic axis. This peculiar “optic pressure” of the light striking area makes it possible “to gather” a wide picture of visual space into a narrower “round slot” in the eye like in a telescope. Indeed, it is so because it is necessary to provide the possibility for these rays to pass even through the narrow but round pupil before their getting on the lens. It should also be noted that the optic pressure of the light striking area occurs, a relative illumination of the latter increases and, in this case, the light rays additionally become almost parallel. In other words, such optic system has a greater narrowing of the light beam but, at the same time, makes them more parallel. However, this is not enough for the optic system of the eye. The nature had to do the following step to press more the light rays into a narrow beam to pass through the relatively narrow pupil. And, upon passing the cornea, to make these rays divergent in order to effectively engage the optic periphery of the lens which provides finer-focusing. It seems to be possible to maximally enhance the effect of the significantly decreased light striking diameter (an optic “pressure” of the incoming ray beam and achieving their parallelism) by means of adding another dispersive surface, a concavo-convex lens, in the optic system studied. Such “a composite lens”, practically, will be identical to the optics of the anterior segment of the eye when the role of the water lens and the glass lens will be fulfilled by the tear film with the epithelium and the cornea, respectively. As it’s seen in the figure, after passing the first lens as the tear film with the epithelium, which essentially disperses but simultaneously “presses” the optic light beam of the lens, there is an additional and yet more significant narrowing of the light striking plane on the inner surface of the cornea (minimal size blue arrow), which, by now, likely, allow to “pass” this pressed beam even through the narrow pupil. And now, it is crucial to explain the following. If the teal film with the epithelium were thicker in the center or had the same thickness throughout, it would be a convergent lens according to the geometric optics laws. However, the thickness of the tear film with the epithelium is always thicker in the periphery than in the center. And due to the optics laws, this lens will be a divergence lens if there is air or another medium with a less index of refraction (IR) along both sides. In the eye, the tear film is in a tight contact with the cornea (without air space); at that, IR of the cornea is higher than that of the tear film by only 0.04. This means that the rays getting on the cornea are already dispersed and pressed by the tear film into the narrow beam, so they will not undergo an additional pressure in the cornea. This is demonstrated in the Figure 11D where the light striking area and diameter are minimal (thick blue arrow). I. e. in the case of the tight contact with the denser cornea, the tear film as a divergence lens cannot fully realize its ability to diverse a light beam and all it can do is to effectively press. And this is the cornea that can realize in full its scattering properties since it borders on the aqueous humor that has a less IR. Let us remind that the corneal thickness is twice less in the center than in the periphery, which is characteristic for the divergence lens. Herewith, on the border of the posterior corneal surface, this maximally narrow light beam will go to the aqueous humor with an IR less by 0.05, so the rays coming out from the retina will be slightly divergent (Figure 11). So, in this case, the cornea itself borders on a less dense optic medium, the aqueous humor, and operates as a lens providing a weak dispersion of the light rays passing through it! Besides, the white light rays will undergo dispersion at the posterior corneal surface and go as divergent and expanding color bands including three basic RGB-bands which can be detected be the macular cones. Actually, this is it of the anterior optic segment of the eye that let the light rays not only pass through the narrow pupil but involve a lens bag peripheral part in the accommodation process since the lens periphery can change RGB-band travel direction in a greater degree than the central flatter part of the lens. We and our colleagues could find only one publication that gives an accurate model how the optic properties of the cornea participate in the terminal optic construction of the eye [25] (citation): “The cornea is a cover of almost equal thickness that is slightly thickened to the periphery. This means that an isolated cornea operates as a weak minus (divergent) lens, which seems a bit unexpected. Computedly, a dispersive power of the isolated cornea of an averaged eye is 5.48 D, and its anterior and posterior focal distances are f = f’ = - 18.25 mm. These numbers refer only to the isolated cornea surrounded by air. In a live eye, the cornea is in completely other conditions. This is only the anterior corneal surface that borders on the air, the posterior surface contacts to the anterior chamber aqueous humor, IR of which is little different from IR of the cornea. Consequently, rays, getting on the eye, pass the cornea that disperse them to the optic axis then enters the aqueous humor almost without changes in their direction. In such conditions, the cornea operates as a strong plus (convergent) lens…”. It should be noted that, in the paper mentioned as most other papers and textbooks, the authors, in fact, take on trust a statement that the cornea is a convergent lens excluding any dispersive effect of the epithelium and the tear film on the general dispersion of the rays coming into the eye. How much these ideas are different from the reality can be understood with our schemes in Figure 11. However, now we should clarify how the physiological mechanism found will function in the conditions of adaptive AL elongation. Let us follow what happens with the human visual system when working at various distances after it has been readjusted by means of AL elongation for priority near work. Figures 12 ABC demonstrate a position of illuminated segments of the macula and intensity of the light striking by RGB-bands at the moment of looking afar and near when high hyperopia in infants gradually transits to high myopia in a result of refractogenesis and when a normal adaptation of its optic axis length, depending on the visual life environment, follows if necessary.
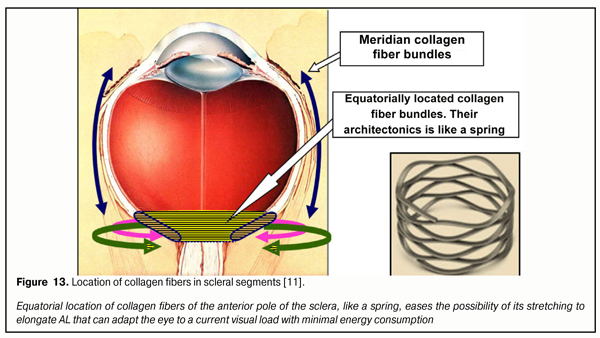
When adaptive elongation of axial length occurs due to priority demand to perform long-term near work under modern display civilization conditions, we lose the possibility to see well afar. However, at other visual distances as well, corresponding changes in functioning of the human visual system are meant to take place. Analysis of Figure 12 can reveal the following. Against the background of young age-related strengthening of eye refraction under 14 years of age and in case when long-term near work is required, there is also additional adaptive LO elongation associated with performing “energy efficiency rule” in the development of biological systems. This normal adaptation process leads to directed changes in anatomic sizes of any body parts involved in order to better adapt to life environment. We are not surprised, for some reasons, at a long neck of a giraffe or extremely long legs of cats that live on the wavy ocean shore: it is easier that way to procure food as branches with leaves and sea fish, respectively. And we do not think of it as of a disease; however, an increase in AL (any degree myopia) is traditionally considered a disease according to International Classification of Diseases (ICD). Emmetropia is called adaptive in Figure 10. And there is something behind that: almost all children are hypermyopes since their early childhood and of them, those whose life environment requires mainly consonant visual distant work will remain the same. You cannot find any myope among reindeer herders over 50 years old. If there is no corresponding environment, there is no myopia either. However, young reindeer herders who actively use displaying “blessings of civilization”, as a rule, become myopes as early as 18 years old [12]. That is why, even emmetropia should be considered adaptive in initially hyperopic eyes in children which adapt to the corresponding life environment. Conclusion: almost all infants are initially adapted to living conditions requiring good distant vision and the possibility of the eye for axial adaptation makes it possible to adapt to other visual life environments to work at medial and close distances. Figure 12A corresponds to comfortable visual distant work with a minimal tension of the ciliary muscle. It demonstrates the divergence of RGB-bands after their passing through the lens, beginning from the period of infancy. In the beginning, the short eye has focus’s position inside the eye for the blue and green wavefronts and beyond the retina for the red one. The scheme shows that the macula gradually moves afar as AL adaptively increases against the background of age-related growth of the eye: physiologically normal mild hyperopia changes into normal but adaptive emmetropia and myopia at school age of 7-14 years old. Herewith, an image is reverse. The lens is maximally flat and, in the normal condition, it is not able to bring the focus out of the retina. It is well seen that as AL gradually increases, the light striking level is redistributed in different segments of the macula. In particular, in emmetropia the width and level of light striking of the ring optic macular segment is sufficient to provide precise focusing for distance when distant work still provides with a necessary signal in this physiological blue ring-optic sight. In a mild myope, this summed signal from the blue cones is weaker and becomes blurrier and starts getting scattered throughout the retina behind the macula that will lead, in high myopia, to a decrease in signal’s threshold electric level required and to impossibility in fact to provide clear distance vision. Likewise, the light striking intensity of RG-bands in the foveola also gets weaker and this impairs the possibilities for clear distance vision. Thus, a decreased area and divergence of RGB excitation bands in the macula results in lost opportunities for clear focusing of the optic system of the eye for distance work when myopic refraction develops. Figure 12B demonstrates long-term loaded near visual work, i.e. when ciliary muscle tension is maximal; in this case, a rounded lens is able no longer to provide the finding of green and red wavefront focuses inside the short eye in which a direct image must appear as in the mild hyperopic eye. It is well seen in the figure, that in hyperopic refraction, the blue light striking band does not get at all in the area of the ring-optic sight; and since there is no blue cone excitation plane, an output electric signal required cannot be created for the brain to send a signal to analyze the image. The figure also demonstrates that, in hyperopia, the signal from the red and green excitation bands in focal plane in the foveola turns almost into a dot, which makes it impossible to provide normal excitation of the red and green cone planes. This corresponds to the known condition “I cannot see”. In emmetropia appears a weakened signal of “the blue optic sight” focusing that allows the brain to send a signal to start analyzing the near image. But in this case, the highest level of “the detection signal” will be observed in mild myopia though the level of the signal from excitation planes will still allow performing analysis of a not-so-good image in the emmetropic eye as well. But continuous near work with the ciliary muscle tension requires much energy or really strong blood supply. And, of course, this requires energy efficiency when an adaptive mechanism for high visual load is put on in order to make a stress visual work more comfortable. In this regard, it is necessary to reduce the tension of the ciliary muscle and by doing so not to lose a functional possibility to analyze the image. The reduction of the CM tension for providing the comfortable visual near work is possible only in case of the further adaptive AL elongation. Figure 12B outlines this state of the visual system. A mean flatness of the lens, when the CM tension is moderate, takes the focal planes of RGB-band wave fronts beyond the retina if the eye is short or mild hyperopic. The blue excitation bands do not get into such eyes; thereto are the eyes with emmetropic refraction. And this is only beginning with mild myopia when physiological sighting can be on and the eye gets the capacity to focus. We should note that a maximal comfortable image in this case will be achieved in the eyes with mild myopia. Thus, the macula must detect not appearance or getting of the focus of each RGB band on its focal plane but it must be set to analyze light striking bands from all RGB-rays rather than to analyze dots (focuses), which is very important. In this regard, all wavefronts of these rays (with each focus is placed on the front’s plane) must always be not behind the retina but in front of it. Only this makes it possible to involve simultaneously the blue cones in the optic ring-sight, which enables to receive a high-quality input optic signal to create a reverse, not direct, image. Theoretical analysis gives an important consequence. To provide high-quality vision, the focuses of RGB excitation bands must always be in front of the retina. So, the statement “the focus is beyond the retina” is incorrect and must be exclude, over time, from the ophthalmic practice: for in this case, it will be impossible to use the optic physiological sight of the eye. Instead of this, it is reasonable to use a statement, which is adequate to the optics of the eye, “a sighting signal” which can be minimal, moderate and maximal. Or, for instance, no sighting signal. These statements absolutely fairly present optical processes that occur in the eye and are important for theoretical and practical optometry. Taking into account the new optic mechanisms found in the eye, it is possible to redefine physiologically these statements which are traditional and familiar for practical optometrists. So, the term under-correction will mean that divergence of the blue excitation band will be so much decreased that the excitation of the blue cones in the macula’s ring-sight will be minimal in case of under-correction, while that will be maximal in case of over-correction. And now, we are aware of the fact that the eye’s focusing mechanism can be connected with the level of divergence of each RGB excitation band in relation not only to each other but to their position on the focal plane in the foveola. This is, in fact, what the total excitation area of all types of cones in the macula will depend on; also, there can occur such conditions when, for instance, not all blue cones which are placed in the fovea centralis surroundings will simultaneously receive the signal of excitation throughout all surrounding segments of this ring-sight. This new look let us define some important hypotheses in vision physiology. These hypotheses were worked out when the metabolic theory of acquired myopia had already been created but they, to our opinion, enabled to come close to understanding possible physiologic mechanisms of the brain-to-ciliary-muscle feedback in eye’s focusing. And, probably, the weaknesses in incremental retinal-defocus theory (IRDT) will be more understood; the basic IRTD statements had been analyzed by us in this journal previously [12]. IRDT authors give top priority to changes of a diameter of light striking spot in different positions of the focus since, in their opinion, this is what the intensity of chemical transmitter production in the retina depends on; these chemical transmitters regulate collagen formation in the sclera. Authors’ greater attention to the diameter (though, to area is more correct) of the light striking spot is a pleasant fact since they as well as other investigators earlier have tried to use a comprehensive approach to the eye considering intraocular systems as a whole. However, we’ve just found out that the matter is not in the diameter of the light striking spots in the macular but in the presence of the optic excitation of the blue cones in the ring-sight. And this is not only for every eye but also for each eye when performing simultaneous binocular work. If the blue cones of the sight are not excited, the brain, likely, cannot activate sending feedback regulation signals to the ocular muscles for eye’s fine focusing. And if the active input signal is absent, then either a visual stimulus is absent or current refraction of the eye keeps out of noticing this stimulus. According to opposing opinion of IRDT authors, here again, the mechanism for retinal production of neurotransmitters which can change collagen formation in the posterior pole of the sclera must be active even at bedtime or when there is no visual stimulus at all, for instance in the eye with the optic nerve cut. And, as we mentioned before, this is obviously incorrect hypothesis of IRTD authors. In 2016, a wonderful analytical review Mysteries of the blind zone and cone-enriched rim at the extreme periphery of the human retina was published by Rozhkova at al. [18] where possible functional properties of the ring with highly concentrated cones were reviewed in details and the authors underlined that the information about this ring had been mentioned in papers by M. Schultze (1866) [35.] and R. Greеf (1900) [31] more than 100 years before. In particular, the review’s authors [18] have noted that “in detailed papers ((R.?W.Williams, 1991 [36]; P.?K. Ahnelt, 1998 [28]) there have been obtained quantitative correlations between the number of the cones and rods in different parts of retinal periphery… It has been shown that, when getting closer to the retinal border, the number of cones not only increases and even exceeds the number of the rods, while in the rest of the peripheral retina the rods are obviously prevailed. As it has been specified the increased cone density is within 1-2 mm area directly bordering on ora serata. According to Williams [36], in the increased cone density ring, maximal cone density is 10000–15000/mm2, which is 3-5 times increases the density in the intermediate periphery, and mean density in the extreme rim of the increased cone density ring with 0.5 mm width is 8 000/ mm2. It is remarkable that a summed number of cones in the increased cone density ring is near 250 000 while the foveal area contains only about 75 000. The modern methods could not only to assess in detail the density of cones in the increased cone density ring but also reveal the presence of cones of all three types (P.?K. Ahnelt, 1998 [28])». The review’s authors also draw attention to the fact “of the presence of the increased cone density ring not only in humans but also in animals, that brings hope of more active research of properties functions in this area due to a wider number of researchers and methods applied” and point that “retinal periphery has advantages in regard of the analysis of locomotion optic flow and its high sensitivity to movements can facilitate to locomotor control accuracy increase, [a] … the periphery plays a critical role in stabilizing the position of the head and body (Bessou et al., 1999 [29])». So, there is also a peculiar “optic ring” of cones on the extreme retinal periphery: in the area of ora serrata; this ring is partially like the “macular optic sighting scope” that we described above. Likely, at the early evolutionary stage, when the lens accommodative mechanism had not been formed, this peripheral optic ring made it possible to organize the initial formed vision of a low quality by means of the presence of RGB-bands in it. Obviously, that “peripheral ring” functionally must be closely connected also with the vestibular apparatus, which can significantly improve control of body position in space even in gravity-free state in the space or in the ocean. Possible anatomic and morphologic likeliness of the nature’s constructive decisions on human vision detection system, which was designed at early and later stages of evolution by forming two “optic sighting rings” both in the macula and in the far retinal periphery, confirms the thesis that the nature replicates successful physiological decisions. According to I.N. Koshits, the formation of the CM-tension-regulating signal to provide high-quality image acquisition is connected not with a usual “absence of image sharpness” mechanism but with a physiological mechanism of comparing the intensity of electrical potential, sizes and inter-position of RGB excitation bands in the macula and on the dome of the retina [5]. It is logically to accept as a hypothesis that accommodation regulation signal from the macula to the brain must be an electric signal which “tells the brain” about inter-position of individual light striking bands created by each RGB excitation band. It should be underlined as well that the green excitation band, likely, is a basic one since, as it was pointed above, the sensitivity of the green cones are many times precedes that of the red and blue cones. It should be noted that if the above mechanism exists then it must have a unique sensitivity. Thus, the brain-to-ciliary muscle functional feedback mechanism must be able to achieve an optimal relative position of RGB light striking planes, which will provide high-quality vision, and, in simple words, optimal eye’s focusing. And this ought to be tested in clinical and psychophysiological experiment. However, let us move beyond. An electrical part of the optic tract (OT) includes: individual visual acuity (IVA) as a generalized quality criterion of the retina as a receiver (retinal discrimination performance and efficacy of mechanism for light’s conversion into the electric signal of the necessary threshold); and axon pathways for electric signal transmission to the neurons of the visual cortex. An optic part of OT from the object to the retina has its transfer function in dependence on the way and parameters of optical correction applied. Apparently, it is rationally to take into account the individual quality of the signal transmission in each optic tract system when choosing rational optic correction (RC). The development of this approach also requires wide clinical investigations which will create a necessary scientific media to work out video-safety criteria. However, the regularities revealed have, in our opinion, another important one for glaucoma pathology. Visual field testing in the eye with glaucoma is, in fact, a test for detecting those ganglion cells in the macula, the axons of which have a greater electrical resistance in the site of their deformation associated with cribriform plate excavation. And precision of examination of the visual fields and their relative position can be improved by means of color-filter that filters, for example, green rays which are able to bring a greater excitation potential to the macula than red rays. Then, probably, a threshold level of a signal coming to the visual cortex neurons will be enough for their excitation. And if it is so, it becomes clear why green spectacles are helpful for glaucoma patients. And to go to even further, it is likely that when changing the position of the wavefront plane using rational optic correction, i.e. shifting the position of RGB light-striking bands to other sites within the macula, it is possible to involve axons, that have not been deformed yet, in the work by maintaining the visual fields by means of formation of a favorable input optic signal. Indeed, today we are able to produce optic lenses in respect of individual visual characteristic of glaucoma patients. So, it is entirely possible that such a direction can be promising; however, this requires advanced clinical investigations. What are other ways, apart from authors’ ideas, that the optical and electrical mechanisms of eye’s focusing can be inter-related with other possible adaptive operative mechanisms of adaptive AL elongation? This question finds the answer in our metabolic theory of acquired myopia, where these mechanisms have been found. Let us describe in details these mechanisms in the relation with eye’s focusing physiological mechanisms revealed above. If there is a vital necessity to perform intensive near work for a long time, the visual system of the human beings and animals will search for a way of such directed changing the anatomical sizes of the eye to perform this visual work with the minimal energy consumption. This is what the normal physiological mechanism which enables lowering the CM tension by means of ocular AL elongation is designed for. When adaptive AL elongation exists, the CM tension will be shifted from maximal to medium/minimal level and, thus, will be favorable for uveascleral outflow pathway. Herewith, physiological conditions of aqueous humor ingredient delivery for collagen reproduction in medial and posterior parts of the sclera will again be restored to the norm; and the energy efficiency law will be executed since the eye will need less blood to perform the same visual work. This is, actually, the essence of the metabolic theory of adaptive myopia. Under the conditions of “display civilization”, initial acquired myopia is obviously useful for a human being and sometimes is required when performing special visual work. However, irrational preventive correction and/or the absence of conditions for comfortable visual work lead to the further adaptive growth of AL by loading or unloading types [3, 4, 7-10, 16, 18, 20]. And, what is very important to understand, adaptive AL elongation can appear even after eye’s refraction is restored to emmetropia by any surgical means. According to our hypothesis, a “switching off” of the adaptive mechanism for eye’s AL elongation is, likely, provided by slight over-correction for distant vision and gentle under-correction for near vision; clinical investigations required to improve this hypothesis have been performed in collaboration with M.G.Guseva, an ophthalmology and optometry specialist [3, 4]. We developed the principle of rational correction as slight optic over-correction for far vision by 0.12 ? 0.25 D and under-correction for near vision by 0.25 ? 0.75 D; this principle, probably, enables to exclude the maximal and minimal levels of the CM tension and to provide more effective retardation of AM and concomitant to it defects on the fundus in comparison with a traditionally recommended under-correction according to Federal clinic recommendations (FCR) of RF: “plus spectacles for continued spectacle wear in risk group (binocular myopic defocus)” (2013) [25,27]. It should be pointed here, that coauthors of FCR RF have also reported of a retarded effect of full correction on the myopia progression [25, 27]; moreover, they have included in the text of FCR the recommendation on using, among other things, night wear orthokeratological contact lenses providing slight over-correction in the macular area and under-correction in the peripheral retina throughout the working day. We should note that FCR have appeared a solid and progressive document permitting everything that is not forbidden! And this is very important for protecting from possible legal actions to optic salon and clinic ophthalmology and optometry practitioners who are not always insured. Morphophysiology. The eye’s posterior pole carcass is anatomically designed in such way that collagen fibers are located here not in meridian but in equatorial direction (T.E. Nikolaieva, 1974) [17] (Figure 13).
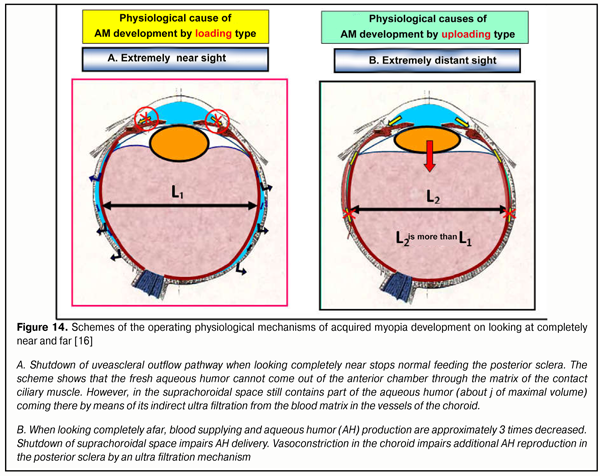
A mechanism for creating functional insufficiency of uveascleral outflow pathway is a basic operating mechanism for environmental conditioning of ocular AL in norm (2001) [20]. In completely near work, the uveascleral outflow pathway (USOP) is interrupted; and in completely distant work, USOP decreases approximately 3 times. A decrease in quantity of metabolites delivered leads to a guided decrease in collagen production intensity, a transient mechanical weakening of the carcass of the medial and posterior scleral parts, and, consequently, a response adequate AL elongation (Figure 14).
Morphophysiologically, scleral architectonic in human beings and in animals is able to guidedly regulate (elongate), in case of vital necessity, the axial length of the eye, which clear confirms our hypothesis that AM is an adaptive process but a disease [15, 16]. To conclude, let us remind you in brief of functional inter-relation of operating physiological mechanisms of adaptive AL elongation in the metabolic theory of myopia. Uveascleral outflow pathway is the only aqueous humor outflow pathway in animals and the leading one in human beings in work at medial and far distances when a trabecular outflow path is not active (Stachs O. at al 2002,2003) [33, 34]. The ciliary muscle as a common operative mechanism of the three regulatory systems (accommodation, production, and outflow) is known to bring into action, at first, accommodation to provide a quality image and, then, aqueous humor outflow (1997) [1]. So, a human being and four kinds of apes evolutionally required the additional formation of the trabecular outflow path for near work when USOP was shut off (2001) [21, 22]. Physiological stages of the operating mechanism of AL elongation in the eye, to our opinion, are as follow [8, 10]: • Initially, a child’s eye has hyperopic refraction, which leads to CM stress when watching near objects; • At these moments, USOP is shut off and aqueous humor nutrition in the intermediate and posterior scleral parts gets worse; • Collagen reproduction in the intermediate and posterior scleral parts worsens at these moments and the carcass of collagen fibers is weakened, which leads to response AL elongation due to IOP action. The eye’s focus is shifted in a position in front of the retina, which allows to reduce CM tension in near work and to lower the energy consumption of the eye. After performing AL adjustment, near work requires the increased CM tension no longer and the volume of necessary blood supplying gets declined but along with this collagen reproduction rates get normalized. In case when visual load is regulated and preventive correction is rational, there are, likely, no conditions for the development of adaptive AM progression [10, 12, 14]. Clinical outcomes (obtained in collaboration) We set investigation tasks in collaboration with M. G. Guseva. Rational correction with special-designed contact and spectacle lenses was performed by M. G. Guseva in 1 704 patients. Control group with spectacle and bifocal under-correction of AM included 788 patients with various degree AM. Follow-up terms were 3, 5, and 7 years. Age: 12-23 y/o; selectively, 28-34 y/o. Display visual load was no less than 4 hours a day in all patients. It was found that early rational correction as well as sparing rational correction depending on the individual sharpen depth (visual acuity) allowed to enhance the efficacy of retardation of AM and its concomitant defects in the fundus (p<0.01) [3, 4]. The efficacy of AM retardation was gradually increased when using regular, bifocal, and progressive spectacle correction of contemporary design (p<0.01) [3, 4]. It was also revealed that regular and progressive contact lens correction of contemporary design had absolute advantage as compared with spectacle lenses (p<0.01); and, among all optical correction means studied, the most effective were night orthokeratological contact lenses (OK) with over-correction at day-time by 0.25 ? 0.75 D (p< 0.001). Rational optical correction with regard to individual visual acuity and a focus’s position on the retina (red-green test) gives the best outcomes in mild over-correction. It should also be noted that the best results of AM retardation were obtained when early correction had been prescribed (3-year follow-up period). This confirms the presence of the adaptive mechanism for AL adjustment for visual load: the earlier we stop its functioning the less degree axial length increases not only in absolute terms but in a significant delay of its own process rates. Practical recommendations for optometrists. In patients with a significantly increased sharpness depth revealed, i. e. with IVA ? 1.25 – 1.5 (~30% of total observed), it is rational to use preserving rational optic correction (RC) which provides a USOP favorable retina-related focal position in near/distant work both in exophoria and orthophoria by means of a rational use of the high individual distinctive ability of the eye. RK for distance. Patients with IVA? 0.8-1.0 and with exophoria require weak over-correction by 0.12 ? 0.25 D, and those patients with orthophoria require taking the focus on the retina. RK for nearness. Patients with IVA? 0.8-1.0 and with exophoria require the same correction as for distance (weak over-correction by 0.12 ? 0.25 D). In patients with revealed IVA ? 0.8-1.0 and with orthophoria, prophylactic spectacles provide under-correction by 0.5-0.75 D, which makes it possible to place the focus on the retina or to provide weak over-correction by 0.12 ? 0.25 D even in case when lens accommodation functional ability of the eye is decreased at the end of the working day against the background that the ciliary muscle gets tired in long-term visual loads. A similar effect of focal position during the working day with USOP favorable CM tension is in domestic spectacle lenses Perifocal with hypercorrection mode in a vertical meridian and in night-wear OC-lenses with day-time weak over-correction by 0.12 ? 0.25 D which have been recommended to use in RF according to FCR-2013. In exophoria, weak under-correction of AM is provided by spectacle progressive lenses with digression + (0.75 – 1.25) D or monofocal SCL in combination with prophylactic spectacles for near vision with the same digression. A brief list of clinical outcomes obtained is as follows: • Myopia progression was the least revealed in the group of children where orthokeratological contact lenses were early prescribed: 0.0 – 0.12 D for 3 years (mild, moderate, and high myopia) • Early prescription of multifocal soft contact lenses with add of 0.75-1.0 D and monofocal soft contact lenses in combination with glasses for near vision +0.75D gives a positive effect of myopia progression braking (mild myopia 0.0-0.12 D, moderate myopia 0.0-0.38 D, high myopia 0.58-1.10 D) for three years as compared to usual myopia progression 1.0 D per year. • Early prescription of glasses with 0.75D addition or bifocal glasses of 0.75 D gave a positive effect of progressive myopia braking (mild myopia 0.04-0.47 D, moderate myopia 0.18-0.48 D, high myopia 1.17-1.21 D) for three years. • Myopia was the most progressive in a group with late prescription of monofocal glasses (mild myopia 0.28-0.82 D, moderate myopia 0.62-1.21 D, and high myopia 1.58-1.95 D) and of bifocal 2.0 D lenses (mild myopia 0.20-0.47 D, moderate myopia 0.60-0.91 D, and high myopia 1.57-1.86 D). This indicates that the earlier rational correction is started, the better myopia braking effect is. • The study revealed that rational optical correction is as follows: weak hyper-correction for distant vision – 0.12-0.25 D and weak under-correction for near vision – 05-0.75 D. Te data mentioned above give a clinical evidence of the main statements of the metabolic adaptive theory of acquired myopia and the following should be noted: • Early full physiologically adequate correction of acquired myopia using orthokeratological contact lenses or contact lenses with prophylactic spectacles for near work (+0.5?0.75 D) in children and adolescents provides apparent braking of its progression or complete stabilization since the focus is shifted beyond the retina, which normalizes metabolic processes in the posterior pole of the eye. • Individual visual acuity as a physiological parameter, which characterizes retinal capacity and enables to receive a better quality output electric signal from the retina, has a significant effect on AM progression: the higher VA the better a braking effect. • Effect of using soft and orthokeratological contact lenses as vision correction means, which facilitates myopia progression braking, is associated with a focal position beyond the retina by (+ 0.12 ? 0.25 D), which, during visual load, makes it possible to avoid AM development by load and unload type. • When maximal individual visual acuity is achieved with orthokeratological full-correction contact lenses, a patient, in fact, becomes an emmetrope or mild hyperope (+ 0.12 ? 0.25 D), which confirms that such prophylactic correction should be implemented into the optometry practice in the conditions of the display civilization. • Application of rational optical correction principles developed makes it possible in some cases to exclude and almost stop developing adverse changes in the eye fundus, which evidences of its significant prophylactic effect. • Prophylaxis of AM development by using rational prophylactic correction in long-term load near work makes it possible to almost completely exclude its progression. • Analysis of clinical data obtained showed that this was the early rational AM correction with night-wear orthokeratological lenses or by modern-designed soft contact lenses that provided the significant positive changes in healthy children and adolescents compared to children who wore spectacles. • Such pathogenically grounded approach significantly slows myopia progression, when accommodation phases are different from extreme ones (look completely at the distance or completely near), enables to involve the whole physiological accommodation amplitude, and normalizes metabolic processes in the anterior pole of the eye through the uveoscleral outflow activation. The clinical data obtained correspond to world trends in searching for effective methods of AM control. For instance, World Health Organization and Brien Holden Vision Institute, at 39 BCLA Conference (Liverpool, 2015), have concluded that “Ortho-k lenses are the most successful myopia control treatment that we have” and proposed to start developing practical recommendations to prevent blindness from myopia including such directions as orthokeratology, 0.5 D correction, focus increase, 0.01 % atropine. We as researchers were also pleased by Russian expert council in refraction and accommodation (ECAR) that recommended in 2015 to start wide clinical researches in RF to detect comparative practical efficacy of complete and incomplete AM correction; and this progressive decision could be made after our results had been published [3, 4, 7, 12, 15]. ECAR recommends carrying out such investigations while we have already obtained practical and promising results with long-term follow-up which significantly confirm the rightness of the metabolic theory of AM which explains physiological advantages of mild over-correction for distant vision in order to effectively control acquired myopia. To conclude, we should note that the adaptive metabolic theory of AM can mark critical aspects as follows: • Rational correction must provide: using the CM functional range in full measure ( the whole accommodation amplitude); full using IVA values as a criterion of distinctive properties of the retina; and qualitative metabolism of the intermediate and posterior parts of the sclera through the USOP favorable position of the focus in norm and in concomitant diseases ( the principle of the “optic” pathway control, 1997) [1]. • Any preventive, functional and treating actions supporting USOP are useful in AM. • AM development exclusion, likely, is possible only in simultaneous solution of two tasks: application of preventive correction and accelerated development of visual work regulations with legislative requirements to videosafety of displays and 3D-technologies, to consideration of a negative effect of modern light sources and e-books in schools, and to a use of ergonomic Cyrillic fonts etc. • The metabolic theory of AM can explain collected clinical data, detect physiological principles of rational correction and develop criteria for comfortable visual work. The metabolic theory evolutionary develops the hypothesis of myopia origin by V.N. Sorokin and E.S. Avetisov (1965) [24] and is a more common theory as compared with modern western theories of retinal defocus and preserving muscular balance. • Until the visual work regulations are designed and considering the reliable prediction of AM dissemination in 5 billion people by 2050, 50% of world population [28], the issues of videoecology and total prevention of blindness take the central stage in fight against the most massive “myopia pandemic” in the history of mankind. In conclusion, it should be noted that the main IRDT hypothesis on the effect of parameters of the visual stimulus on the axial growth of the eye in myopia coincides with the metabolic theory; however, IRDT authors, to our opinion, could have not found immediate and adequate operating mechanisms of AL elongation. These mechanisms have been explained by the metabolic adaptive theory of acquired myopia. Taking into account the conclusion given in two previous papers [8, 12] and the theoretical and clinical analysis performed, we can conclude: 1. The analysis of the organization and structure of the input optic signal coming to the macula and the analysis of characteristics of macular morphological functional structure revealed that, in the macula of the human eye, there is an optic ring-sighting scope that has a high concentration of the blue cones and that can provide a quick eye’s focusing at the required area of the visual space. 2. The functional interaction of the excitation planes in the ring-sighting scope of the each eye allows the brain, on the basis of comparative analysis of their electrical parameters and geometrical position in the cortex, to create a required signal to control all muscles of the both eyes in order to provide their binocular work and effective focusing at the required space area. 3. The physiological hypothesis of eye’s focusing proposed made it possible to get closer to understanding of, likely, the main secret of human accommodation system control: creating a feedback signal from the brain to the ocular muscles with the help of analysis of excitation plane position in the ring-sight of the macula can be the leading physiological mechanism of the eye’s focusing. 4. Analysis of morphological functional structure of the retinal periphery in the site of ora serrata revealed a certain similarity in the organization of the peripheral “ring-sight” and the ring-sight in the macula. Their functional interaction has yet to be studied. 5. Adaptive elongation of the axial length when performing the stressful visual work is connected with performing the law of energy efficiency in biological system development and makes it possible to provide the maximal feedback signal for effective eye focusing including that in binocular work. 6. After the incoming white light rays are dispersed into the spectrum at the inner corneal surface, in the eye for further formation of the effective optic signal there created not light scattering circles but the basic excitation bands of three colors: red, green, and blue (RGB-bands), to which the cones of the macula are adjusted. 7. The optometric term of “focus-defocus” is not completely correct and adequate to the optic system of the eye since the presence of axial aberration keeps out of combining the focuses of all RGB-bands in one common plane. 8. The state “the focus beyond the retina” suggests the presence of the direct, not reverse, image and the state “the focus on the retina” must lead to visual field excitation in the macula in the single focal point, which, in fact, cannot create the image of electrical excitation plane of the notable area which is required for analysis. 9. The correct term is not “a focus of the eye” but “a focal plane in the foveola", in which, to provide comfortable vision, there must be placed simultaneously green (basic) and red excitation bands with parameters which depends on the static and dynamic refraction of the eye and on the distance from the object-stimulus observed. 10. The blue excitation bands, simultaneously coming into the ring-sight of each eye, allow accommodation control system to form an adequate feedback signal for ciliary muscle tension regulation and, in consequence, for USOP intensity regulation that regulates the level of metabolism in the medial and posterior sclera. 11. Rational optic correction will give the possibility to form such an optic signal from RGB-bands in the macula which will not give the physiological mechanism of the adaptive AL elongation to actively function due to the recovery of normal metabolism in the sclera of emmetrope and myope at initial stages. 12. Under-correction, in our understanding today, means a significant decrease in intensity or absence of light striking from the excitation plane in the ring-sight in the macula; while over-correction means their adequate increase that enables to clearly provide focusing on the part of the visual space required. Acknowledgements We would like to show our gratitude to: Marina G. Guseva, a highly professional ophthalmology and optometry specialist, for the provided opportunity to co-participate for 7 years in her dissertation research on “Studying the efficacy of rational optical correction for acquired myopia stabilization” that proved clinically the efficacy and scientific rationale of the metabolic theory of adaptive myopia and gave important recommendations for practical optometry; Leonid I. Balashevich, one of our real teachers, Honored Scientist of RF, principle researcher of St. Petersburg branch of Fyodorov Eye Microsurgery Complex, Dr. Sc. (Med.), Prof., for invaluable help when the first paper was prepared for publishing and for creation of amasingly favorable scientific atmosphere which made it possible to develop the metabolic theory of the adaptive myopia and to reveal, for the first time, new effective physiological mechanisms of the eye’s focusing; Feliks N. Makarov, our co-author of many years, Head of Neuromorphorogy Laboratory at Pavlov Institute of Physiology RAS, Dr. Sc. (Med.), Prof., for a number of important comments on morphological and functional structure of the human retina; Svetlana V. Alekseenko, a leading researcher of Pavlov Institute of Physiology RAS, Dr. Sc. (Biol.), for help in understanding the mechanisms of excitation plane interaction in the cortex that provides binocular spatial vision; Alla A. Ryabtseva, Head of MONIKA ophthalmic clinic, principle non-staff ophthalmologist of Moscow region government, Dr. Sc. (Med.), Prof., for many-year opportunity to share the data obtained within annual scientific conferences of ophthalmologists in Moscow region on issues of myopia.
References 1. Volkov VV, Kotliar KE, Koshits IN, Svetlova OV, Smol'nikov BA. [Biomechanical characteristics of interactions between the drainage and accommodation regulatory systems of the eye in health and in contusion lens subluxation]. Vestn Oftalmol. 1997 May-Jun;113(3):5-7. In Russian. 2. Guseva MG. [Analysis of spectral features of the light reflected from the eye fundus in some fundus diseases. Abstracts of scientific practical conference of young scientists, Leningrad Institute of Expertise and Organization of Labour for the Disabled]. L.: 1992; 37. Russian. 3. Guseva M. G., Svetlova O. V., Koshits I. N. [Stabilization of the acquired myopia in children with the aid of contact lenses from the standpoint of the metabolic theory of myopia]. Oftalmol Zh.2011;5:29-37. Russian. 4. Guseva MG, Svetlova OV, Koshits IN. [On a choice of physiologically-based rational correction for stabilization of acquired myopia in children]. Glaz. 2012;1:12-7. Russian. 5. Deinego VN, Kaptsov VA, Balashevich LI, Svetlova OV, Makarov FN, Guseva MG, Koshits IN. [Prevention of ocular diseases in children and teenager in classrooms with led light sources of the first generation. Analytical review]. Ross. Detsk. Oftalm. 2016;2:57-72. Russian. 6. Zharov VV, Nikishin RA, Egorova AV et al. [Clinical assessment of the accommodation state using computered accommodography]. [Biomechanics of the eye - 2007: proceedings of scientific conference]. M.: MNIIGB im. Gelmgoltsa; 2007. 3-8. Russian. 7. Koshits I. N., Svetlova O. V. [Discussion issues of acquired myopia]. Ophthalmol Zh. 2012;6:111-122. In Russian. 8. Koshits I.N., Svetlova O.V. Adaptive myopia. Part 2. New ideas on physiological mechanisms of eye’s focusing. J.ophthalmol.(Ukraine).2017;1:38-50. 9. Koshits I.N., Svetlova O.V. Adaptive myopia. Part 1. New ideas on physiological mechanisms of eye’s focusing. J.ophthalmol.(Ukraine).2016;6:45-54. 10.Koshits IN, Svetlova OV. Formation of the adequate length of eye and the metabolic theory of the pathogenesis of acquired myopia. Oftalmol Zh. 2011;5:4-23. Russian. 11.Koshits IN, Svetlova OV. [Ontogenesis of formation of necessary length of the eye and metabolic theory of myopia pathogenesis]. Biomechanics of the eye- 2007: proceedings of scientific conference]. M.: MNIIGB im. Gelmgoltsa; 2007. 13-33. Russian. 12.Koshits IN, Svetlova OV. [Ontogenesis of formation of necessary length of the eye in childhood and metabolic theory of myopia pathogenesis]. Glaz. 2007;6(58):16-31. Russian. 13.Koshits IN, Svetlova OV, Guseva MV, Makarov FN. [Priority directions of the fight against acquired myopia: theory and practice]. Glaz. 2013. 6(82): 12-7. Russian. 14.Koshits IN, Svetlova OV, Gorban AI. [Physiological peculiarities of actuating mechanisms of accommodation and development of Helmholtz theory. (Monograph)]. Izd. Dom. SPb MAPO; 2014. 93 p. Russian. 15.Koshits IN, Svetlova OV, Makarov FN. [Acquired myopia as a classic case of prevalence of accommodation over outflow]. [Binocular and oculormotor disorders: proceedings of scientific practical conference in Helmholtz Moscow Research Institute]. M.;2007:135-7. Russian. 16.Koshits IN, Svetlova OV, Makarov FN. [Metabolic theory of progressive myopia. Proceedings of XII Congress of Ukrainian Ophthalmologists]. Odessa; 2010. 257. Russian. 17.Nikolaieva TE. [To a comparative structural inferiority of accommodative muscles and sclera in moderate severe myopia. Myopia: collection of papers of scientific practical conference in Helmholtz Moscow Research Institute]. M.; 1974. 49-51. Russian. 18.Rozhkova GI, Belokopytov AV, Gracheva MA. [Mysteries of the blind zone and cone-enriched rim at the extreme periphery of the human retina]. Sensornyie sistemy. 2016;4(30):263–281. Russian. 19.Svetlova OV. [Functional peculiarities of the interaction between the sclera, accommodation and drainage systems of the eye in glaucoma and myopia pathology]. Author’s thesis for Dr Sc (Med). M.; 2010. 55p. Russian. 20.Svetlova OV, Koshits IN. [Biochemical aspects of possible common causes of inherited and acquired myopia. Myopia, disorders of refraction, accommodation, and oculormotor system: proceedings]. M.:MNII im. Gelmgoltsa; 2001: 77-78. Russian. 21.Svetlova OV. [Regulatory biomechanism of uveascleral outflow in the human eye]. Ophthalmology between two ages: proceedings. SPb: VMA; 2001: 207-208. Russian. 22.Svetlova OV, Koshits IN., Drozdova GA. [Pathophysiological peculiarities of interaction of aqueous humor outflow and accommodation mechanisms in myopia and glaucoma. (Monograph)]. Izd. Dom SPb. MAPO; 2014. 170 p. Russian. 23.Sivukhin DV. [Physics guideline. The 3rd Edition. In: Optics]. M.: Fizmatlit, MFTI; 2002. 792 p. Russian. 24.Sorokin GA, Avetisov ES. [On a new hypothesis of myopia origin. In a book “Proceedings of scientific conference, dedicated to 90th anniversary of V.Filatov”]. Kiev; 1965: 34-9. Russian. 25.Tamarova RM. [Optical devices for examination of the eye]. M.: Meditsyna; 1982. 176p. Russian. 26.[Federal clinic recommendations. Diagnostics and treatment of myopia in children. Interregional public organization “Association of ophthalmologists”]. Moscow; 2013. 49p. Russian. 27.[Federal clinic recommendations. Diagnostics and treatment of myopia in children]. Ross. Pediatricheskaia Oftalmologiia. 2014;2:49-62. Russian. 28.Ahnelt P?K. The photoreceptor mosaic. Eye;1998;12:531–540. 29.Bessou M, S?verac Cauquil A, Dupui P, Montoya R, Bessou P. Specificity of the monocular crescents of the visual field in postural control. Comptes rendus del’Acad?mie des sciences. S?rie III, Sciences de la vie. 1999;322(9):749–757. 30.Brien A, Holden at al. American Academy of Ophthalmology. 2016; www.aaojournal.org. 31.Greef R. Mikroscopische Anatomie der Sehnerven und der Netzhaut.- Eds A. von Graefe, T. Saemisch. Handbuch der gesamten Augenheilkunde. Leipzig: Verlag von Wilhelm Engelmann;1900. B.1:1–212 32.Gullstrand A. Einfuhrung in die Methoden der Dioptrik des Auges des Menschen. Leipzig: S. Hirzel Verlag; 1911. 33.Stachs O, Guthoff R, Ludvig K. Monitoring the human ciliary muscle function during accommodation. Current aspects of human acommodation II. Heidelberg, 2003. 105–18. 34.Stachs O, Martin H, Kirchhof A, Stave J, Terwee T, Guthof R. Monitoring accommodative ciliary muscle function using three dimensional ultrasound. Graefe`s Arch. Clin. Exp. Ophthalmol. 2002; 240:906-12. 35.Schultze М. Zur anatomie und physiologie der retina. Archiv f?r mikroskopische Anatomie.1866; 2 (1):175–286. 36.Williams RW. The human retina has a cone-enrichedrim. Vis. Neurosci. 1991;6(4):403–6.
|