Adaptive myopia
Part 2. New ideas on physiological mechanisms of eye’s focusing
1Koshits I.N.
2Svetlova O.V.
1Petercom-Networks / Management Systems Consulting Group Cl. corp., St. Petersburg, Russia;
2The I.I. Mechnikov Northwestern State Medical University, St. Petersburg, Russia.
We reviewed morphology and physiology of macular zone of the eye and analyzed, adequately to the optics laws, an input optic signal from the white light coming into the foveola. The white light beam are dispersed at the outer corneal surface: these are not circles of scattered light but excitement bands of red and yellow cones in the foveola, and of blue cones on its circular periphery.
We reviewed in detail the authors ' hypothesis on the presence of the fovea of the functional mechanism of “comparison of the location and intensity of the excitement fields of red, green and blue cones. This organization of the input optical signal allows the brain to create a response signal to control tone of ciliary muscles to install the required refractive power of the lens. Executive mechanism of the eye’s focusing is associated with operation in the fovea of the optical ring-sight consisting of dark blue cones with maximal possible concentration. Functional peculiarities of interaction of the rings-sights in binocular work of both eyes are identified.
This hypothesis may have already helped detect the physiological mechanisms of the eye’s focusing and, apparently, in the future, would develop criteria for video security and visual work rules to fight against the pandemic of myopia. The work of found executive eye focusing mechanisms is considered by the authors in relation to their proposed hypothesis on metabolic theory of adaptation of myopia (2001). Until the rules of visual work are absent, the problems of video ecology and total prevention of acquired myopia are on the first place in the fight against the myopia pandemic.
Key words: adaptation, acquired myopia, metabolic theory, early correction, visual acuity, video ecology, regulations for visual work, bands of excitation in the macula, mechanisms to control accommodation, axial length, and focusing.
An extensive analysis of hypotheses and actuating mechanisms of optic axis lengthening in incremental retinal-defocus theory (IRDT) has been performed in our previous article [15]. Despite the fact that IRDT development, historically, has been a move forward, our analysis has revealed incomplete validity of IRDT hypothesis in regard to actuating mechanisms proposed and shown the absence of clear physiological criteria as for adequate optical correction choice to fight effectively against world myopia pandemic [37]. Besides, it has remained unclear in IRDT what a physiological entity of a traditional term of “image focus” is and what connection it has with light-striking area in focus or hyperopic/myopic defocus.
This made us to advance a study, started 20 years ago, on search for still undiscovered eye’s focusing mechanisms and their interactive functioning with actuating mechanisms of adaptive increment of axial length (AL).
1. Ideas on eye’s focus
Do we understand clearly what the eye’s focus is? Indeed, the traditional term of eye’s focus in ophthalmology is rather intuitive so far since it has not been determined by clear physiological and quantitative criteria as yet. Practical optometry still does not have methods for objective diagnostics in vivo which can assess, for instance, the distance (mm) before and behind the retina in which the focus is at the moment.
A discrete step in sets of trial ocular lenses equals 0.12 or 0.25 D and it may be too big to specify a focus site in the eyes, for instance, with individual depth of vision equal to 1.25 D and higher. This is because the quality of the retina as a receiver is significantly higher in these eyes but we do not always take it into account when choosing corrective lenses. In optometry practice, in general, the term of focus intuitively conforms to equal image sharpness against a red or green background and this is only an axiom.
Figure 1 shows extreme conditions of the optic system of the eye when, in the condition of a hard visual work near, a completely rounded lens cannot level the outgoing, for instance, of the red wavefront behind the retina when using the whole spectrum of ciliary muscle activity.
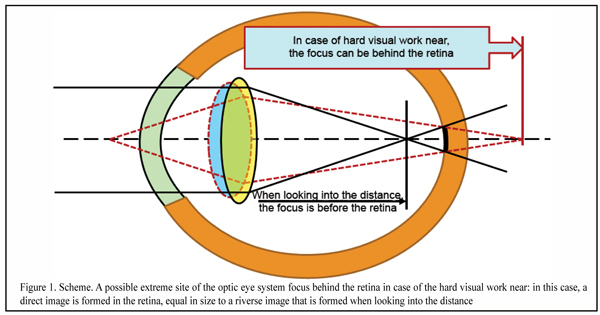
In case of the intensive visual work near, a direct image, not inversed (reverse image), will be formed in the retina. But we traditionally believe that this is only the reverse image that is always formed in the eye; and so, it gets unclear what happens to the image in over-correction.
However, when focus is accurate in the retina, in the macula there will be projected not a bar or circle of light scatter but only one dot, turning now into the reverse and then into direct image. And the dot is a minimal excitation signal which is not completely good. It is obvious that a reliable detection system cannot operate on the basis of such a weak and questionable input optic signal.
And this can mean that an optometric mantra “beans converge before and behind the retina” does not stand up to criticism and something has to be done with it. As well as with a term of “hyperopic defocus” that supposes the presence of the direct image in the eye. And if to go on, a traditional term of “focus behind the retina”, basically, makes no sense since a traditional reverse image can be formed only if focus is before the retina even in extreme phases of accommodations.
However, we all know that we can analyze even inverse picture in the cinema or in life after short-term adaptation. Hence, the brain is not set for reverse image analysis only but can easily analyze direct images. This simple idea can step, in some cases, out of “red flags” of the creed that there is only a reverse image in the eye. Indeed, it can be supposed that the brain is able to determine the moment when the direct image turns into reverse; so, could this determine the moment for the onset of a sharp image?
A bit later we will describe in details morphophysiological retinal structure that enables the eye to use dispersion of a white-light beam at the posterior surface of the cornea. And now, it is important to understand that after the dispersion of the white-light beam into spectrum, the most important for the eye are dispersive bands of three basic colors which can be detected by the macula: red, green, and blue (RGB-bands).
A scheme of the dispersive front after white light passing through the cornea is given in Figure 2. We should draw the attention that, of the spectrum, we marked only three basic bands: red, green, and blue; in other words, only those bands that can be detected by corresponding RGB-cones of the macula.
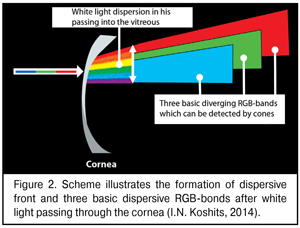
As it can be seen in Figure 2, the white light gets dispersed at the posterior corneal surface and a width of partially overlaid dispersive bands of different colors, including RGB-bands, increases on their way to the lens since the cornea, essentially, is a scattering lens. For the lens between the extreme phases of accommodation can markedly shift along the axial length from 0.3 to 1.2 mm and, in significant ciliary muscle tension, even to shift down to 0.25-0.38 mm [12,14,38], the width of three basic RGB-bands on their arrival at the lens will be changed in some way.
After input light bean is refracted in the cornea and is dispersed at the posterior corneal surface, dispersive colored wavefronts will pass through the aqueous humor of both chambers and through the lens, by now, without dispersion but will have the second additional angle deviation in the lens for each RGB-band, according to its wave frequency and length.
According to optics laws, focal planes of wavelengths of RGB-colors are always separated in the eye, and a focal plane of red light is always closer to the lens, whilst the farther is a focal plane of red light. This condition is called axial (longitudinal) chromatic aberration.
However, according to which of these three RGB-light focal planes should the eye “choose” the focus? In fact, these are three “focuses” rather than one. This means that a traditional concept of the presence of the one single focal plane in the eye is, at least, incorrect: three dispersed planes of wavelengths cannot be combined in one plane “by definition”; and this longitudinal aberration on the optical axis objectively exists and we use it, by the way, when detecting in practice the “accurate” focus with a red-green duochrome test.
And even if we define a concept of focus, for instance, according to one of three RGB planes, what, whereas, will under- and over-correction mean? And if we traditionally suppose that the image is reverse in the eye, so, we have to accept that RGB-rays passing out along the lens can deviate as much as to cross the optic axis before the retina and, thereafter, they will, likely, part in space with no chance to get into the macula area. Let’s find out where, in fact, focal planes of wavelengths of RGB-lights will be placed on the optic axis.
Index of refraction (IR) is ?1.376 in the cornea, 1.386 in the lens, 1.336 in the vitreous and aqueous humor. This means that white light passing from dense medium (cornea) to thin (aqueous humor in the anterior chamber) should undergo not only dispersion but the first angle change of every component of its spectrum.
Refraction in the optic system of the human eye ranges within 52.5-71.5 D (at average 60.0D) in adults [34, 8] and equals 77.0-80.0 D in children [10]. So, optic focus (F) even in newborns minimally is F=100cm/80D=1.25 cm and can place before the retina. According to V.K. Verbitskii, refraction power of a reduced eye is 58.82 D, i. e. focus of the reduced eye is 100/58.82=1.72 cm. This is almost 17.4 times less than the axial length (AL) = 30mm in the eye with high acquired myopia (AM) and 14 times less than in the emmetropic eye, for instance, with AL = 24 mm.
In other words, traditionally, reduced focus of the eye, generally, always places before the retina, and to place it behind the retina, even in the emmetropic eye, an additional splitting lens with refraction power of no less that D=100cm/2.4mm=41.7 D is required. Glasses or contact lenses with such a high optic power are not used in practice. We should note that these early ideas that summarized centuries-long research on the eye’s optics have made it possible to move forward rather than block the further development of optometry theory. If these ideas of highly and really reputable authors are correct, we have to accept that focus always places inside the eye.
Thereafter, a question about the rightness of optometristis’ statement that “focus places behind the retina” or whether we very well realize what exactly focus is becomes not an idle inquiry. Perhaps, we should consider in the eye not traditional optics of RGB lights passing when a reverse image is formed but, also, optic regularities in position of RGB-bands of excitation in the direct and reverse image, which are different from the bean in having a significant and changeable width in the macular area and are not connected with a term “focus on the retina”? And these brand new “physiological doubts” of us will considerably help in further reasoning on the rightness of “focus of the eye” term.
It is obvious that human visual detection system can either neutralize axial aberration or use it. Let us assess these possibilities to try to understand what the focus of the eye is. What if using this common term in ophthalmology is not only incorrect but wrong? And this question itself is really heretical by now. However, a new knowledge at a new round of experience is the best cure for errors.
2. Velocity of light passing inside the optic structure of the eye and distances between focal planes of RGB-lights
Here, we should clarify if a wavefront (WF) of each RGB light gets to the macula simultaneously in any axial length or some of them do not get to the fovea at all in extreme phases of accommodation. A less stickler for detail can jump this section with elementary calculations within school physical optics, however, we ask to draw your attention to section’s conclusions.
Velocity of light in water was measured by W. Weber and F. Kohlrausch in 1856 and specified by J. С. Maxwell in 1856 [2]. It has been found to be 4/3 times less than that in vacuum. It has been found that refractive index of water “n” is nR=1.331 and nG=1.335 for extreme red and green lights, respectively, in a spectrum of visible light and is nV = 1.342 for extreme violet lights [2,32,33,38].
The difference in velocity of red VR and violet VV lights of the spectrum, according to the formula V = "c"/"n" , where light velocity in vacuum с = 3 х 108 m/c, will be as follows: VR = 3•108 / 1.331 = 2.25 •108 (m/s), and VV= 3•108 / 1.342 = 2.235 •108 (m/c), i. e. VR – VV = 0.015 •108 (m/c). This is a relative velocity that WF of red lights will “run away” in the eye from violet light WF when passing through the aqueous humor and vitreous after white light dispersion at the posterior corneal surface. And when passing through the aqueous ocular structures, green light WF will “run away” from red light WF, respectively, with a relative velocity VR – VG = 2.25 •108 – 3 /1.335•108 = (2.25 – 2.247) •108 = 0.003 •108 (m/c), which is 5 times slower than from violet light WF.
This means that the distance between red and green WF planes after passing through the optic media of the eye will be significantly smaller than that between red and blue. This also means that excitation bands (EB) of red and green WFs will always place in the macular area closer to one another than those of red and blue WFs. Moreover, absolute path difference of spectrum lights’ WF, i. e. their relative gap to red light, is connected with a different coefficient of their retardation in the “composite” optical medium of the eye, in other words, with velocity of the passage of each light along the axial length through the anterior and posterior chambers, lens, vitreous, and retinal thickness. In further calculations, braking capacity of the fovea structures for RGB-light WF will be set equal to aqueous media of the eye, which will not break common reasoning.
It should be noted that when RGB lights pass from the cornea to the macula through anterior and posterior chamber humor as well as through the vitreous, their wavefronts are retarded according to individual refractive index; whilst when passing through the lens they are retarded due to the higher refractive index of the lens. In other words, the lens can bring a significant value into the common braking path of WF of each of RGB lights. Let us estimate it.
To our best knowledge, there are no direct investigations on refractive index of red and violet colors in the lens. However, it turned to be possible to calculate it according to findings of other investigations. In particular, it is known that refractive index of red and violet lights in heavy flint (HF) glass is IRHF red light=1.6444 and IRHF violet light=1.6852, respectively [33, 45]. It is also known that refractive index of white light in heavy flint glass is IRHF white light=1. 6475 [33], and refractive index of white light in the lens (L) is IRL white light=1. 386 [38, 45]. IR ratio for rays of white light is IRL white light / IRHF white light = 1.386 / 1.6475 = 0.84. This IR ratio must be remained both for violet and red lights both in the lens and aqueous media of the eye.
Thus, being aware of refractive indices of RGB-rays in water, it can be easy to calculate their refractive indices in the lens. Therefore, refractive indices of red, green, and violet rays in the lens are IRLR=1.331/0.84=1,584, IRLG=1.335/0.84=1.589, and IRLV=1.342/0.84=1.597, respectively. This eases to calculate maximal values of “braking paths” in the lens immediately in extreme phases of accommodation for each WF of RGB-rays.
Velocity of each of the RGB-rays in the lens is VLR= 3•108 / 1.584 = 1.893 •108 (m/c), VLG= 3•108 / 1.589 = 1.888 •108 (m/c), and VLV= 3•108 / 1.597 = 1.878 •108 (m/c), respectively. The lens, when looking near, is completely “rounded” and has the maximal thickness of 5.0 mm. On passing through such a lens, the braking path difference, avoiding routine calculations, is 5.0 mm – 4.986 mm = 0.014 mm between red and green WF and 5.0 mm – 4. 958 mm = 0.042 mm between red and violet WF. When looking into the distance, i.e. when the lens is flat with 3.5 mm thickness, the difference is 0.01 mm between red and green WF and 0.03 mm between red and violet WF. In other words, braking capacity of the optic medium of the lens for RDB-light WFs in extreme phases of accommodation differs by 0.004 mm between red and green WFs, and by 0.012 mm between red and violet WFs.
To our opinion, it is difficult to create in the macula such a highly-sensitive physiological mechanism to be able to assess significantly rather big values of WH deviation from each other along the AL in various accommodation phases within 0.004-0.012 mm. This is because the linear size (height) of light-sensitive outer cone segment that contains light-sensitive membrane discs with iodopsin (it is a kind of biological accumulator) does not exceed 50 micron=0.0005 mm [46]. Therefore, there must be another highly-sensitive physiological mechanism to assess a shift of RGB-light wavefronts along the axial length. Let as estimate such a possibility.
When emmetropic eye axial length (LEE) from the cornea to the retina averages 24.0 mm and maximal thickness of the lens is 5.0 mm, optic signal path length through the anterior and posterior chambers and the vitreous is 19.0 mm.
Red WF passes this path for ТR=LEE/VR=19.0•0-3 (m)/2.25•108 (m/c) = 8.444 •10-11 (c). This is time for violet WF to pass the length LF = ТR • VF = 8.444 •10-11 (c)•2 .235 •108 (m/c) = 18.87 mm. Thereafter, the absolute path length difference (gap) of violet WF from red FW in emmetropic eye is maximally 0.042 mm (in the lens) + 0.13 mm (in the aqueous media) = 0.172 mm. So, the lens can contribute up to 25% to difference in braking path length of violet and red wavelengths. And this is a great deal, which, likely, enables the eye fine-focusing.
Likewise, in green light, when emmetropic eye axial length in the aqueous media is LG = ТR • VG = 8.444 •10-11 (c) • 2.247 •108 (m/c) = 18.97 mm, the absolute path difference, i.e. ssumarized gap of green WF from red WF when passing the rounded lens and the aqueous media of the eye is 0.014 mm (in the lens) + 0.03 mm (in the aqueous media) = 0.044 mm.
Here, it should be noted that alterations in the lens geometry participate in the release of accommodation mechanism not only due to changes in angle of refraction of RGB-rays but also due to noticeable changes in braking rates of wavelengths when their thickness is changed. This, to our opinion, makes it possible to open, perhaps, a “new chapter” in more modern IOL constructions. It also indicates undoubted prospects of investigations by O. Nishi on refraction and accommodation recovery in presbyoptic period with placing a presbyocapsule containing inert refractive fluids into the lens sac [42, 43]. This technology can make it possible to preserve the capability of the filled IOL to finer-focus as compared to rigid IOL due to additional regulation of braking path difference in RGB-rays when its thickness is changed, especially in the extreme accommodation phases.
In the eye with high AM, for instance, with AL = 30 mm, red WF passes the path (30 mm- 5 mm) = 25 mm along the axial length through the aqueous medium of both chambers and the vitreous for ТR = LEE / VR = 25.0 •10-3 (m) / 2.25 •108 (m/c) = 11.111 •10-11 (c). This is the time for violet WF to pass the length LV = ТR • VV = 11.111 •10-11 (c) •2 .235 •108 (m/c) = 24.83 mm. Thereafter, the absolute path difference (gap) of violet WF to red WF in the high AM eye is maximally 0.042 mm (in the lens) = 0.17 mm (in the aqueous media) = 0.212 mm. Likewise, for green light, when its path length in the aqueous media of the emmetropic eye is LG = ТR • VG = 11.111 •10-11 (c) • 2.247 •108 (m/c) = 24.96 mm, the absolute path difference, i. e. summarized gap of green WF to red WF is 0.014 mm (in the lens) = 0.04 mm (in the aqueous media) = 0.054 mm.
The calculations given mean that the contribution of lens thickness itself has a significan effect on the summarized value of path difference of RGB-rays from the cornea to the retina both in the emmetropic eye and in myopia of any degree. However, the main purpose of the lens which is to alter the angle of deviation of RGB-rays by changing the curvature of its anterior and posterior surfaces will be also achieved.
Let us subtotal. Maximal values of gap of violet and green WF focal planes in regard to red WF when lens thickness is 5.00 mm in the emmetropic eye (AL = 24 mm) and in high myopia eye (AL = 30 mm) equals 0.172 mm / 0.044 mm and 0.212 mm/ 0.054 mm, respectively. In other words, in the eye with any degree myopia, the absolute value of gap of blue WF focal plane to red does not exceed 0.212 mm, and of green WF to red is 0.054 mm. A scheme for placement of RGB WF focal planes in the macula in the emmetropic eye and in high myopia is given, respectively, in Figure 3.
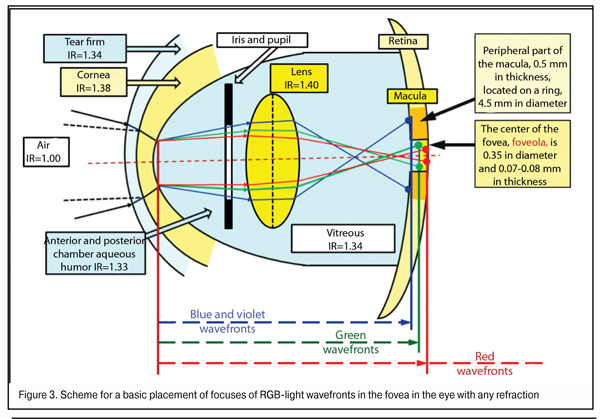
In the norm, the thickness of the fovea at the edge of its central part, the foveola, is maximal, 0.5 mm, and that is 0.07 to 0.08 mm in the centre. This means that even when AL is abnormal, in all cases of red WF placement on the posterior surface of the foveola surround 0.5 mm thick, all three RGB spectrum components will necessary get to the fovea area (Fig. 3). That’s why, if, in the human eye, the red focal plane does not get “behind the retina” at the further edge of foveola thickness, the violet-blue focal plane will always reach the posterior relatively thick surround of the fovea rather than will get, for instance, before it.
In other words, in even high myopia, a relative placement of green and red WF planes will always appear simultaneously in the foveola thickness. And it is clear now that, in the normal and myopic eye, when binocular work is adequate in both eyes, red and green excitement bands are present in the fovea simultaneousely and always, which can be a clear input control signal in finer-focusing due to accommodation-controlling brainwaves.
Prof. Balashevich has hypnotized that different color rays can excite cones at different parts of their anatomic length, varying the level of output electrical potential. After all, the cones, essentially, consist of a set of “flat biological accumulators” connected only with one current-conducting axon. A physiological mechanism of the finer-focusing may also be connected with resolution ability of the foveola, i.e. with a diameter and a number of cones to receive red and green light. As it was already said approximately 30 % of people have visual acuity (VA) more than 1.25 D, and in some people have VA of 2-2.5 D. VA is known to be exponentially higher in some birds.
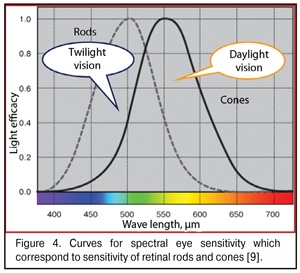
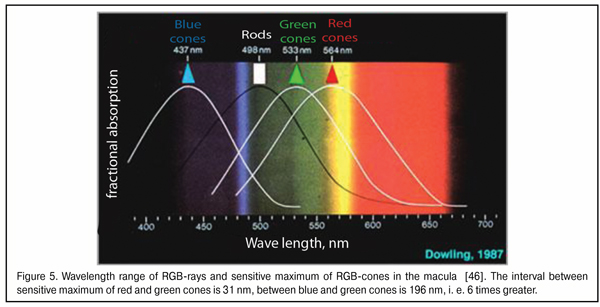
As said above, the “gap” between red and green WF focal planes does not exceed 0.05 mm in the emmetropic and mild myopic eyes. When foveola thickness is 0.07-0.08 mm, red and green excitement bands will always be found simultaneously in it. Bearing in mind that the sensitivity of green cones providing daylight formed vision is tens of times higher than that of red and blue cones (Fig. 4), it is logical to assume that the green excitement band is basal in the eye and this is the green band that is taken as “a starting band” in eye’s focusing.
It should be also noted that a wavelength is minimal in a blue light bean and maximal in that of red light. The wavelength of the green light is in the intermediate position between them. Blue and red rays carry the maximal and minimal energy, respectively. So, when passing through a lens, the blue rays are refracted more than the red ones. But what is also important, the difference in the angle of deviation between the red and green rays is smaller than that between green and blue because of closer coincidence in their wavelength ranges (Fig. 5). In other words, the red and green light striking bands in the macula will be initially closer to one another than the green and blue bands.
So, it is likely, that a traditional term of focus has to be reconsidered in ophthalmology since we are at a new round of knowledge. And a new term of focal plane of excitement bands which is adequate to the visual system of the eye has to be introduced instead; the distance of focal plane from the cornea is constant in all accommodation phases: this is the foveola where not focal points but GR-bands will be placed. Why these are bands but not circles of confusion, we’ll try to explain in our next paper.
However, in which way does the eye get focused if there is “no focus” but is a focal plane in the eye? Obviously, there must be some other physiological mechanisms which have not been described previously. Especially since formed binocular vision is provided by two eyes. And it is absolutely clear that any physiological “focusing” mechanisms in the human visual system, i.e. visual signals in focal planes in both eyes, must be cerebrally interrelated.
3. Morphophysiology of the retina and mechanisms of primary processing of the background image optical signal
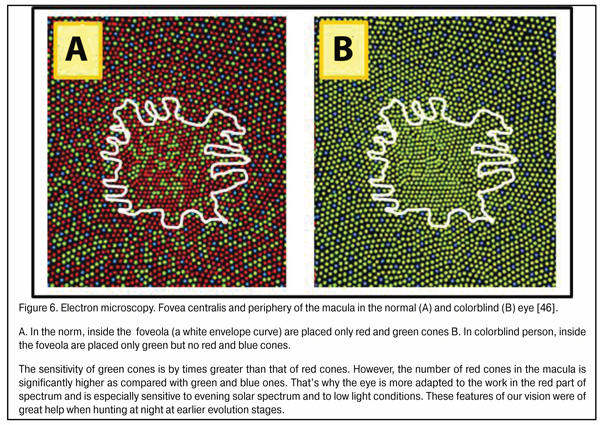
There is a good reason that cones are sensitive to three spectral colors: blue (440-480 nm), green (510-550 nm), and red (620-770 nm). This helps us create an image color-grade of high quality. But there is another important issue. In the fovea are located only red and green cones; while blue cones located in its periphery are essential not only for color vision but, likely, can participate in general system providing good-quality vision as a reserved subsystem securing accommodation control at half-lights and at night (Fig. 6) the site in the foveola without blue cones are outlined.
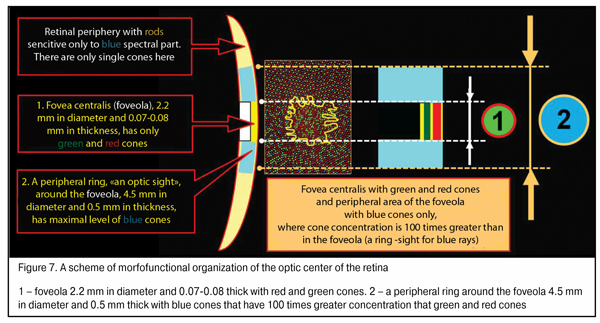
We should note that the maximum density of blue cones occurs in a ring 4.5 mm in diameter (stereo angle 180) around the fovea 2.2 mm in diameter which is 100 times greater than that of red and green cones; and the thickness of ring periphery about the foveola is 0.5 mm here [46]. This ring, likely, plays a leading role in eye’s focusing physiological mechanism and in what follows we will consider how it operates. Besides, in the retina there are horizontal and amacrine cells in, respectively, outer and inner plexiform layers supporting horizontal bindings between all retinal cone and rod excitement fields.
This means that in macula occurs a highly-sensitive mechanism to detect mutual position as well as relative rates of RGB-excitement fields on the cupola and in the macula. In plain language, the retina can estimate the color, disposition and size of light spots coming into the eye from a detail in general background that the brain marked to sight.
An organizing scheme of the optic center of the retina shown in Figure 7 will be of use for understanding how an input optic signal is organized in accommodation control system functioning. Such a morphological construction of the fovea is as it were created for the functioning of the highly effective physiological mechanism for the comparison of the disposition, area and intensity of excitement fields with red, blue and red light according the area of the fovea.
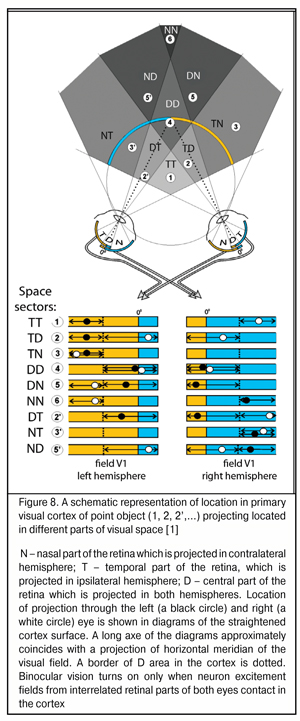
To date, it has been found that in primary visual cortex of each cerebral hemisphere, except the contralateral visual hemifield there is an ipsilateral part. Therefore, the information from the central parts of visual fields of the eyes is received in both hemispheres. The width of this area of “double” occurrence in the central horizontal meridian of visual field is 3-4 angular degrees in non-human primates and in humans and increases up to 30-40 angle degrees at extreme superior and inferior periphery [38, 41, 44]. Such projecting is provided by nasal-temporal overlapping areas in the retina in which both ipsilaterally and contralaterally projecting ganglion cells are located.
Such interrelated functioning of both eyes’ retinas has been described in details by S.V. Alekseenko, Dr. Sc. (Med.) (2014) who has found that a similar mechanism to assess relative positions of excitement fields is used in the nature, in particular, in the organization of binocular neurons of visual cortex in cats (Fig. 8) [1]. The distance between input cells of these neurons conditions their depth setting in space. Herewith, focusing the objects located in a focal plane is performed by binocular neurons, inputs of which are located next to each other, i.e. nearly adjoin. This indicates that physiological mechanisms set to detect the distances between the excitement fields which occur in stimulation of different eyes have already been used in the nature.
Repeat occurrence of signals, received from the part of cones which are “criss-cross” connected with retinas, in different hemispheres of the brain is a most important morphophysiological feature of the visual system and, likely, enables to use parameters of nasal-temporal overlapping of RGB-excitement fields for binocular focusing and beyond. In simple words, a nasal half of the retina of one eye is rigidly connected with a temporal half of the retina of the other eye, which has long been known.
Electric excitement fields from retinal halves of each eye occur repeatedly in different hemispheres and their assessment leads to adequate interrelated mixing and dilution of their optic axes to obtain the possibility of binocular vision, first of all, to specify the distance to a potential danger. Besides, such functional interaction of different retinal halves of both eyes makes it possible to get bigger angles of observation in peripheral (not binocular) vision functioning and detect immediately “which side” the danger comes from.
It should be also noted that the area of binocular field of vision with both eyes is oriented in the vertical meridian and is always 70-80% less than the area of the fields of vision of every eye separately (see Figures in [1]). To understand this, to our opinion, is of special necessity for an ophthalmology practitioner at early stage of open-angle glaucoma detected in a patient when the area of the binocular vision field is still normal and, in fact, “masks” a decrease in its own vision field area in the eye with initial glaucoma: the patient has excellent binocular vision and glaucoma process is still invisible but occurs so far.
It is critical to note that morphological structure of the retina in the macular area differs significantly from the structure in its farther periphery. It can be schemed as a two-layer model: with two layers of rods in the macula though, obviously, there are no such layers; and each neighbor rod is connected by its own axon with different but neighboring neuron excitement fields from different retinal halves (greenish area in Figure 9A).
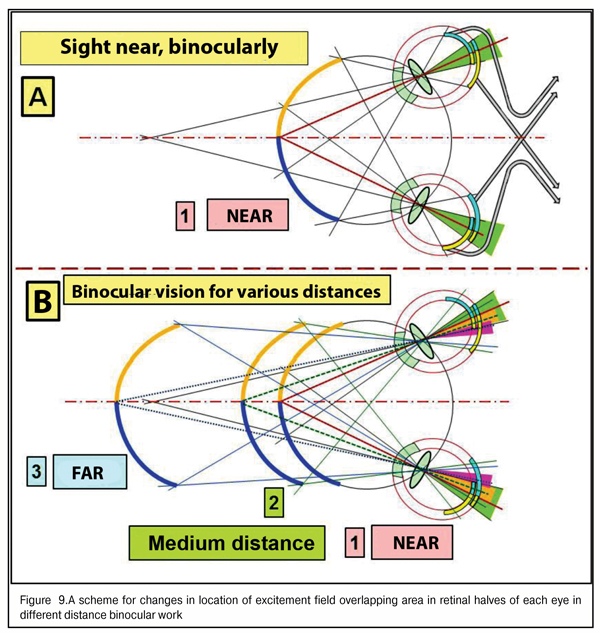
The maximal area of “overlapping” of rod excitement field in the foveola corresponds to precise binocular focusing of both eyes to an object marked out from the visual background for detailed sight (case 1A in Fig. 9). Also, Figure 9 shows that when the object sighted distances gradually afar, the overlapping areas get narrower (cases 2 and 3B in Fig. 9, colored yellow and violet), excitement areas in the retinal periphery decreases, which leads to a decrease in general level of electric excitement of cone fields in the macula and rod fields in the peripheral retina.
And, indeed, the brain will send a control signal to extraocular muscles to perform a corresponding interrelated rotation of both eyes to achieve the maximal area of overlapping, in other words, to achieve a control signal of maximal level. Conclusions: providing the binocularity of vision supposes the presence of the physiological focusing mechanism as a kind of “scope sight”. But what kind of scope is it and how does it work?
We supposed that a powerful excitement signal from blue cones, placed in the ring about the foveola and having its maximal concentration in there, is a limiting “detection” signal to start the finer-focusing actuating mechanism: the brain understands that, having such a position of the focus border in the eye, the fovea can “catch” all RGB-bands after dispersion of white light in the cornea. Thereafter, after primary “sighting”, it is possible to start a process of finer-focusing, binocular so far, in three-dimensional space through analyzing an input signal in the fovea from GR-lights in a “two-layer pie”. We can determine this round focal border of blue lights as a mechanism of preliminary search and sighting.
Figure 10 shows an operating scheme for search and sighting when the focus object distances from the eye. Here, in much more details than in Fig. 9 can be seen changes and a decrease in the light striking sector, in the retinal periphery and in the macular, of excitement fields in the foveola when shifting the gaze from near to the distance. It’s very well seen how simultaneously works the binocular three-dimensional “scope sight” which is located in the retina of each eye as a peripheral ring about the foveola and which has the maximal concentration of blue rods responding to the most powerful part of the spectrum, a blue excitement band.
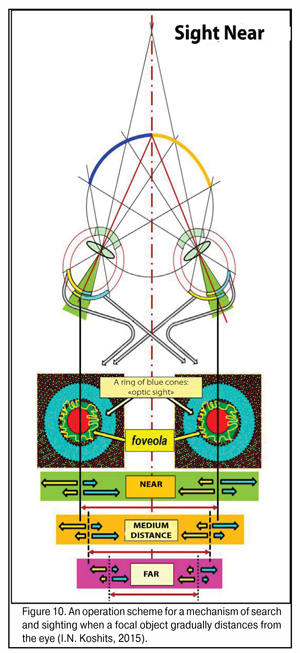
Such morphofunctional structure of the macula enables “to sight” reliably even in law light conditions when there is a strongest violet-blue spectral part in the input light. Evolutionary choice of the blue spectral part for reliable functioning in any light conditions and at any time of day is a reflection of the fact that, likely, the eyes of humans and animals initially were perfectly adapted for night vision including a low lit upper ocean.
We should also note that, in particular, two areas of optic signal overlapping in the “layers” of the fovea centralis are, likely, first al all, responsible for finer-focusing: for the best vision, the excitement level of the green and red rods of the overlapped concentric foveola “layers” must be maximal. In this regards, the brain using the feedback system must simultaneously send adequate electric control signals to ciliary and extraocular muscles.
Indeed, in order this physiological mechanism to operate immediately and reliably, it needs to receive a best-organized input optic signal. Traditionally, this is supposed to be circles of light scatter. However, if it is so and if this shape of the input optic signal is optimal for its preliminary assessment in the macular and retinal periphery? It required a special analysis.
According to the hypothesis of I.N. Koshits, since the fields of excited RG-rods are connected horizontally and it is possible to compare their areas and intensity of optic excitement in the fovea, the retina, likely, can differentiate the cases of contacting, overlapping or gapping of RGB-excitement bands coning into the eye at the moment of operating with far, medium, and near distances.
To understand physiological features of the feedback mechanism that provides focusing through signals to ciliary muscles, we need to know the placement of borders and the color of the input optic signal coming in the macula as RGB-bands.
We should note that the electromagnetic wave passes from one optic medium to the other, frequency ? (and period Т) remains unchanged; this is only a wavelength ? to be changed. In particular, when light passing from vacuum to the media with refractive index n, the wavelength decreases n times [32].
Figure 11 demonstrates a scheme for gapping, contacting or overlapping of the excitement fields in the macular in different accommodation phases. Morphophysiological structurte of the macula makes it possible to assess with a high quality and almost immediately the placement of excitement bands to each other for the brain to produce a feedback signal to the ciliary muscle to form a corresponding tension.
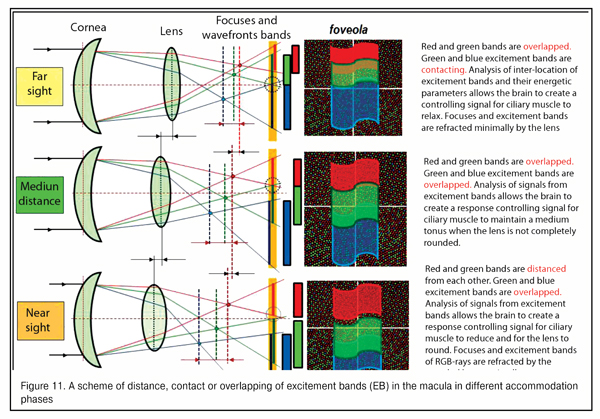
We should point that this is preliminary assessment of interrelated disposition of RGB-excitement bands in the macular rather than “image sharpness” is a qualitative and highly-sensitive input signal for the whole accommodation control system. Optical and physiological features of this signal as well as the interrelation between this input optic signal with our metabolic adaptive theory of acquired myopia (2001) will be described in our next paper.
References
1. Alekseenko SV. [Organization of binocular system for three-dimensional space representation]. Glaz. 2014; 1(95): 28-30. Russian.
2. Vafiadi VG, Popov YuV. [Speed of light and its meaning in the science and technology]. Minsk; 1970. 43 p. Russian.
3. Verbitskii VK. Optic features of the eye. Eye Diseases. L.: Prakt. Meditsina; 1930. 75-94. Russian.
4. Volkov VV, Kotliar KE, Koshits IN, Svetlova OV, Smolnikov BA. [Biochemical features of interaction of accommodative and drainage systems of the eye in norm and in contusion lens subluxation]. Vestn. Oftalmol. 1997;3:5-7. Russian.
5. Guseva MG. [Analysis of spectral features of the light reflected from the eye fundus in some fundus diseases. Abstracts of scientific practical conference of young scientists, Leningrad Institute of Expertise and Organization of Labour for the Disabled]. L.: 1992; 37. Russian.
6. Guseva M. G., Svetlova O. V., Koshits I. N. [Stabilization of the acquired myopia in children with the aid of contact lenses from the standpoint of the metabolic theory of myopia]. Oftalmol Zh.2011;5:29-37. Russian.
7. Guseva MG, Svetlova OV, Koshits IN. [On a choice of physiologically-based rational correction for stabilization of acquired myopia in children]. Glaz. 2012;1:12-7. Russian.
8. Dashevskii AI. [Refraction and accommodation of the eye. Guidance for eye diseases. Book 1]. M.: Medgiz; 1962. 252-263. Russian.
9. Deinego VN, Kaptsov VA, Balashevich LI, Svetlova OV, Makarov FN, Guseva MG, Koshits IN. [Prevention of ocular diseases in children and teenager in classrooms with led light sources of the first generation. Analytical review]. Ross. Detsk. Oftalm. 2016;2:57-72. Russian.
10. Kovalevskii EI. [Ophthalmology. Textbook]. Meditsina; 1995. 480 p. Russian. .
11. Koshits I. N., Svetlova O. V. [Formation of the adequate length of eye and the metabolic theory of the pathogenesis of acquired myopia]. Oftalmol Zh.2011;5:4-23. Russian.
12. Koshits IN, Svetlova OV. [Ontogenesis of formation of necessary length of the eye in childhood and metabolic theory of myopia pathogenesis]. Glaz. 2007;6(58):16-31. Russian.
13. Koshits IN, Svetlova OV. [Development of Helmholtz's theory of accommodation according to studies of additional accommodation mechanisms]. Vestnik RAMN. 2003;2:3-12. Russian.
14. Koshits IN, Svetlova OV. [Modern visions of Helmholtz's theory of accommodation: work-book]. SPb: ID SPbMAPO; 2002. 30p. Russian.
15. Koshits I.N., Svetlova O.V., Guseva M.G., Balashevih L.I., Makarov F.N., Egemberdiev M.B. Actuating mechanisms of optic axis growth in the incremental retinal defocus theory. J.ophthalmol.(Ukraine).2016;6:43-57.
16. Koshits IN, Svetlova OV, Guseva MV, Makarov FN. [Priority directions in the fight against acquired myopia: theory and practice]. Glaz. 2013;6(82):12-7. Russian.
17. Koshits IN, Svetlova OV, Gorban AI. [Physiological peculiarities of actuating mechanisms of accommodation and development of Helmholtz theory. (Monograph)]. Izd. Dom. SPb MAPO; 2014. 93 p. Russian.
18. Koshits IN, Svetlova OV, Makarov FN, Guseva MV. [Rational correction is an effective way to stabilize acquired myopia. Abstracts of IX All-Russian scientific practical conference “Fedorov Memorial Lectures”]. M.; 2011. 104-6. Russian.
19. Koshits IN, Svetlova OV, Makarov FN. [Acquired myopia as a classic case of prevalence of accommodation over outflow]. [Binocular and oculormotor disorders: proceedings of scientific practical conference in Helmholtz Moscow Research Institute]. M.;2007:135-7. Russian.
20. Koshits IN, Svetlova OV, Makarov FN. [Modern concepts about executive mechanisms of accommodation and Helmholtz theory. Part 1. Physiological and biomechanical peculiar properties of the accommodation system]. Glaz. 2012;2(84):11-20. Russian.
21. Koshits IN, Svetlova OV, Makarov FN, Guseva MV. [On physiological and biomechanical postulates of new accommodation theories]. Glaz. 2013; 3 (82): 15-21. Russian.
22. Koshits IN, Svetlova OV, Makarov FN. [Metabolic theory of progressive myopia. Proceedings of XII Congress of Ukrainian Ophthalmologists]. Odessa; 2010. 257. Russian.
23. Koshits IN, Svetlova OV, Makarov FN, Shilkin GA. [Classification of actuating mechanisms of “objective” accommodation in human being]. Ross. Detskaia Oftalmolologiia. 2012; 4: 28-36. Russian.
24. Landsberg GS. [Optics. The 6th edition]. M.:FIZMATLIT; 2003. 848 p. Russian.
25. Svetlova OV. [Functional peculiarities of the interaction between the sclera, accommodation and drainage systems of the eye in glaucoma and myopia pathology]. Author’s thesis for Dr Sc (Med). M.; 2010. 55p. Russian.
26. Svetlova OV, Koshits IN. [Biochemical aspects of possible common causes of inherited and acquired myopia. Myopia, disorders of refraction, accommodation, and oculormotor system: proceedings]. M.:MNII im. Gelmgoltsa; 2001: 77-78. Russian.
27. Svetlova OV, Koshits IN. [Interaction of main intraocular fluid outflow pathways with accommodation mechanism: textbook]. SPb.: ID SPbMAPO; 2002. 50p. Russian.
28. Svetlova OV, Koshits IN. [Classification and interaction of accommodation mechanisms in the human eye]. Biomechanics of the eye – 2000: proceedings. M.: MNII im. Gelmgoltsa; 2002: 117–119. Russian.
29. Svetlova OV. [Regulatory biomechanism of uveascleral outflow in the human eye]. Ophthalmology between two ages: proceedings. SPb: VMA; 2001: 207-208. Russian.
30. Svetlova OV, Koshits IN., Drozdova GA. [Pathophysiological peculiarities of interaction of aqueous humor outflow and accommodation mechanisms in myopia and glaucoma. (Monograph)]. Izd. Dom SPb. MAPO; 2014. 170 p. Russian.
31. Svetlova OV, Koshits IN., Kugleev AA. [Physiological peculiarities of ciliary muscle in guided refractive interventions]. VII Congress of ophthalmologists of Russia: proceedings. Moscow; 2000: 287. Russian.
32. Sivukhin DV. [Physics guideline. The 3rd Edition. In: Optics]. M.: Fizmatlit, MFTI; 2002. 792 p. Russian.
33. Taylor B, Parker W., Langenberg D. [The Fundamental Constants and Quantum Electrodynamics. M; 1972.
34. Tron EZh. [Changeability of elements of optic system of the eye and its meaning for clinic]. L.; 1947. 354p. Russian.
35. Shapalov S, Shelepin IuE, Miliavskaia TI et al. [Guidance on diagnostics, expertise, recovery of visual capacity in persons of air and dispatcher staff of civilian aviation]. M.: Vozdushnyi transport; 1990. 104p. Russian.
36. Shapovalov SL, Miliavskaia TI, Ignatiiev SA. [Formation and course of myopia in adults]. M.: MIK; 2012. 199p. Russian.
37. Brien A. Holden at al., 2016, American Academy of Ophthalmology; www.aaojournal.org.
38. Gullstrand A. Einfuhrung in die Methoden der Dioptrik des Auges des Menschen. Leipzig: S. Hirzel Verlag, 1911.
39. Hess C. Die Refraction und Akkommodation des menschlichen Auges und Anomalien.- Albrecht von Graefes Arch. Ophthal. 52:143-74,1901.
40. Fukuda Y, Sawai H, Watanabe M, Wakakuwa K, Morigiwa K. Nasotemporal overlap of crossed and uncrossed retinal ganglion cell projections in the Japanese monkey (Macaca fuscata). J. Neurosci. 1989; 7(9): 2353-73.
Crossref Pubmed
41. Marzi C.A., Mancini F., Sperandio I., Savazzi S. Evidence of midline retinal nasotemporal overlap in healthy humans: A model for foveal sparing in hemianopia? Neuropsychologia. 2009; 3 (47): 3007–11.
Crossref Pubmed
42. Nishi Okihiro, Nishi Kayo. Accommodation Amplitude After Lens Refi lling With Injectable Silicone by Sealing the Capsule With a Plug in Primates.- Arch. Ophthalmol.,1998; 10 (116): 1358–61.
43. Nishi Okihiro, Yamamoto Naoki, Nishi Kayo, Nishi Yutaro. Contact inhibition of migrating lens epithelial cells at the capsular bend created by a sharp-edged intraocular lens after cataract surgery / pmid:17531703. Cataract Refract Surg.,2007; 33 (6): 1065–70.
Crossref Pubmed
44. Reinhard J., Trauzettel-Klosinski S. Nasotemporal overlap of retinal ganglion cells in humans: a functional study. Invest. Ophthal.Visual Sci., 2003; 44: 1568-1572.
Crossref Pubmed
45. http://www.nkj.ru/archive/articles/6674/ (Science and life, retarded light).
46. https://traditio.wiki/ Retinal central fovea











