Comparison of expression of molecular markers of peripheral blood lymphocyte activation in patients with small uveal melanoma and healthy controls
S.I. Poliakova, Dr Sc (Med)
L.N. Velichko, Cand Sc (Med)
A.V. Bogdanova, Cand Sc (Biol)
I.V. Tsukanova, Junior Researcher
Filatov Institute of Eye Disease and Tissue Therapy
Odessa, Ukraine
E-mail: inna.sister@mail.ru
Background: Organ-saving treatment is indicated for patients with small (i.e., ? 3 mm in prominence) uveal melanomas (UM), that being the initial stage of the disease. Association between the level of activation of the immune system and the progress of UM has been demonstrated in recent decades. Investigation of the expression of molecular markers of lymphocyte activation is important and required for predicting tumor responses to various treatments and choosing the most effective treatment for the disease.
Purpose: To investigate the expression of molecular markers of peripheral blood lymphocyte activation in patients with small uveal melanomas prior to treatment.
Methods: We compared the expression of molecular markers of peripheral blood lymphocyte activation in 16 patients with small uveal melanoma (prior to treatment) and 44 healthy controls (donors).
Results: Prior to treatment, patients with small uveal melanoma were found to have elevated expression of molecular markers of peripheral blood lymphocyte activation compared to healthy controls (р < 0.0002).
Conclusion: In uveal melanoma patients at the initial stage of the disease (i.e., with tumors ? 3 mm in prominence), the functional activity of immune competent cells of the body in response to tumor development was found to involve the following: (a) activation of lymphocyte IL-2 receptors (CD25+), (b) increased indices of lymphocyte activation and proliferation (CD38+, CD45+, CD150+), immunoglobulin production (CD150+), and intercellular adhesion (CD54+) and apoptosis (CD 95+), and (c) induction of cytokine secretion (CD7+).
Key words: small uveal melanoma, molecular markers of peripheral blood lymphocyte activation.
Introduction
Organ-saving treatment is indicated for patients with small (i.e., with a prominence of 3 mm or less) uveal melanoma (UM), that being the initial stage of the disease. However, tumor response to this treatment may vary, with small UMs being often poorly responsive to treatment and tending to progress and metastasize, and larger ones tending to regress [1]. Association between the level of activation of the immune system and the progress of UM has been demonstrated in recent decades [2-5]. Understanding the nature of the interaction between tumor cells and immune competent cells is crucial for achieving the most effective treatment. Therefore, investigation of the expression of molecular markers of lymphocyte activation is important and required for predicting tumor responses to various treatments and choosing the most effective treatment for the disease [6].
The purpose of the study was to investigate the expression of molecular markers of peripheral blood lymphocyte activation in patients with small uveal melanoma prior to treatment.
Materials and Methods
We compared the expression of molecular markers of peripheral blood lymphocyte activation in 16 patients (4 men and 12 women; mean age, 55.4 ± 11.1 years) with small uveal melanomas (prior to treatment) and 44 healthy controls (donors; 18 men and 26 women; mean age, 55.4 ± 11.5 years). Study group patients with small uveal melanoma had tumors with a mean prominence of 2.09 (0.83) mm with 0.4 mm and 3.0 mm as a minimal and maximum values, respectively; a mean tumor length was 5.86(4.24) mm with its minimum and maximum as 1.0 mm and 12.0 mm, respectively. No chronic and metastatic conditions were revealed in the study group patients at baseline. Neither were acute or chronic diseases revealed in healthy controls.
The expression of molecular markers of peripheral blood lymphocyte activation was determined immunohistochemically [7]. Venous blood samples (5 ml) were taken prior to treatment. The immunophenotyping panel of monoclonal antibodies (MCAb) included the antibodies responsive to СD7+, CD25+, CD38+, CD45+, CD54+, CD 95+, CD150+ antigens [8].
All statistical analyses were performed by STATISTICA 9 software Quantitative variables were presented as means (M) with standard deviations (SD) and compared using analysis of variance (ANOVA) (Fisher test and Newman-Keuls test).
Results and Discussion
Table 1 shows the data used to compare the expression of molecular markers of peripheral blood lymphocyte activation between patients with small uveal melanomas (prior to treatment) and healthy controls (donors). It is seen from the table that in patients with small uveal melanomas (prior to treatment), the absolute and relative expression levels of CD25+, CD38+, CD45+, CD54+, CD 95+, CD150+ were significantly higher than those in healthy controls (p < 0.0002). Additionally, in patients with small uveal melanomas, the absolute expression levels of CD7+ (a costimulatory molecule that reflects immune cell response to uveal melanoma more than the molecules mentioned above) were higher than those in healthy controls, although the relative expression levels were low (p < 0.0001).
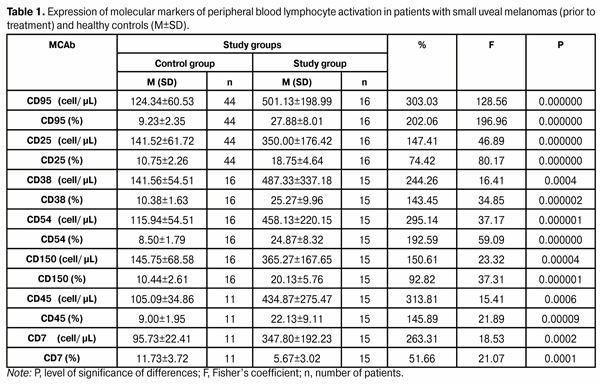
Elevated expression of CD25+, CD38+, CD45+, CD54+, CD 95+, CD150+ on peripheral blood lymphocytes in patients with small uveal melanomas is evidence of activation of immune competent cells of UM patient’s body in response to tumor development. Activation of lymphocyte IL-2 receptors (CD25+), induction of cytokine secretion (CD7+), and increased indices of lymphocyte activation and proliferation (CD38+, CD45+, CD150+), immunoglobulin production (CD150+), and intercellular adhesion (CD54+) and apoptosis (CD 95+) were observed.
Although CD7+ is an activation marker, its expression may be reduced in high activation level of CD4+ CD25+ regulatory T cells (Treg) that are important for regulation of immune response and may play a key role in induction of tumor-associated T-cell tolerance by suppressing the T-cell response. With tumor growth progressing, a 40 per cent expansion of this Treg lymphocyte subset has been found to occur, which results in inhibition of immune response [4].
Therefore, lymphoid cells exhibit high functional activity in UM patients at the initial stage of the disease. This suggests that UM response to therapy at the initial stage of the disease must be rather marked. However, UM patients differ in treatment effect, and it has been proved that their immune system not only fails to reject the developing tumor, but sometimes facilitates tumor progression [8].
In UM patients, favorable prognostic values of immunological and molecular parameters of lymphocytes have been identified which facilitate positive outcomes of combined modality organ-saving treatment for uveal melanoma [6]. In this connection, it is important to understand the course of anti-tumor immune response to various therapeutic modalities in UM patients at the initial stage of the disease, and how this response influences the therapeutic treatment effect of these modalities (transpupillary thermotherapy, in particular). Our further studies will be focused on these issues.
Conclusion
In uveal melanoma patients at the initial stage of the disease (i.e., i.e., with tumors ? 3 mm in prominence), the functional activity of immune competent cells of the body in response to tumor development was found to involve the following: (a) activation of lymphocyte IL-2 receptors (CD25+), (b) increased indices of lymphocyte activation and proliferation (CD38+, CD45+, CD150+), immunoglobulin production (CD150+), and intercellular adhesion (CD54+) and apoptosis (CD 95+), and (c) induction of cytokine secretion (CD7+).
References
1. Terent’ieva LS, Kotova VA, Shambra VV. [Variation of radiation sensitivity of melanoma as a function of tumor size and irradiation conditions]. Oftalmol Zh. 1993;1:5-7 Russian
2. Blom DJ, Luyten GP, Mooy C. Human leukocyte antigen class I expression. Marker of poor prognosis in uveal melanoma. Invest Ophthalmol Vis Sci. 1997 Aug;38(9):1865-72.
3. Vetter CS, Lieb W, Br?cker EB, Becker JC. Loss of nonclassical MHC molecules MIC-A/B expression during progression of uveal melanoma. Br J Cancer. 2004 Oct 18;91(8):1495-9.
Crossref Pubmed
4. Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005 Apr;6(4):345-52.
Crossref Pubmed
5. M?kitie T, Summanen P, Tarkkanen A, Kivel? T. Tumor-infiltrating macrophages (CD68(+) cells) and prognosis in malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2001 Jun;42(7):1414-21.
6. Velichko LN. [Expression of molecular markers of peripheral blood lymphocyte activation in uveal melanoma patients with various efficacy of organ-saving treatment]. Oftalmol Zh. 2013;5:9-13 Russian
Crossref
7. Gluzman DF, Sklyarenko LM, Nadgornaya VA, Kryachok IA. [Diagnostic immunocytochemistry of tumors]. Kiev: Morion; 2003. 156 p. Russian
8. Berezhnaia NM, Chekhun VF. [Immunology of malignant growth]. Kyiv: Naukova Dumka; 2005. 790 p. Russian

