J.ophthalmol.(Ukraine).2017;1:20-24.
|
https://doi.org/10.31288/oftalmolzh201712024 Functional status of platelets in type 2 diabetes patients showing no diabetic fundus changes Introduction
Diabetes mellitus (DM) and diabetes-related vascular complications are still associated with premature mortality and incapacitation of working age individuals and thus substantially affect demographic trends [1]. The changes developing in a DM patient are characterized by alterations in endothelial and nerve cell metabolism which are accompanied by disorders of various tissues and organs. Diabetic retinopathy (DR) is one of the commonest complications of DM and may lead to a persistent loss of visual function. Early detection of retinal disorders is therefore essential for secondary prophylaxis of DR. Even the latent and early stages of diabetic angiopathy are accompanied by morphological fundus changes. At the same time, in clinical and laboratory studies, these changes are identified if only they are manifested in some way, e.g., through retinal hemorrhage, thrombosis, edema, soft and hard exudates and neovascularization. A search for informative indicators of the DM-related changes (including diabetic angiopathy) should be therefore linked to the investigation of potential causes of microcirculation impairment. Type 2 diabetes mellitus (DM2) is known to be accompanied by endothelial dysfunction and impairment in the platelet phase of hemostasis [2]. Since the platelet proaggregation status is a predictor of microcirculatory abnormalities, we might hypothesize that platelet aggregation (PAG) analysis in DM2 patients will allow us to come closer to identification of informative indicators of the risk for the development and progress of DR. We believe that investigation of the pathogenesis of abnormalities in microcirculation (in particular, dysfunction of the platelet phase of hemostasis) is an adequate way of solving the problem. The study purpose was to investigate the functional status of platelets in type 2 diabetes patients showing no diabetic fundus changes. Materials and Methods Thirty eight DM2 patients (38 eyes) manifesting no diabetic fundus changes were included in this case-control study. Key exclusion criteria included the presence of age-related macular degeneration, clinically significant media opacities, a recent history (6 months or less) of ocular surgery or laser treatment, neovascular glaucoma, degenerative myopia, amblyopia, a history of refractive surgery, or a history or current use of intravitreal injections. Patients were also excluded if they had a history of cardiac infarction, stroke, obstructive pulmonary disease, renal insufficiency, or cancer. At baseline, all patients included in the study were seen by endocrinologist and nephrologist. The ophthalmological examination included visual acuity assessment using intelligent refractor RT-5100 (Nidek, Gamagori, Japan) and chart projector CP-770 (Nidek); non-contact tonometry (NT-530; Nidek); perimetry with the Humphrey Field Analyzer (Carl Zeiss Meditec AG, Jena, Germany); keratometry and refractometry with the Refractive Power/Cornea Analyzer ARK-1000 (OPD-Scan II, Nidek); anterior eye slit lamp biomicroscopy (BQ 900; Haag-Streit, K?niz, Switzerland); retinal wide-angle biomicroscopy (Super Pupil XL; Volk Optical Inc., Mentor, Ohio); gonioscopy and ophthalmoscopy with contact and noncontact lenses (Volk Optical Inc.); optical coherence tomography (Optovue, Inc., Fremont, CA); and fundus photography (the Early Treatment Diabetic Retinopathy Study (ETDRS) seven standard fields) and fluoresecent angiography with the fundus camera TRC-NW7SF (Topcon, Tokyo, Japan). Diabetic retinopathy was diagnosed based on the modified ETDRS classification. The study eyes of all patients were graded as no visible diabetic abnormalities (grade 0). The control group was age-matched with the study group and included 10 healthy individuals who underwent diagnostic evaluation and who had no clinical or laboratory evidence of cerebrovascular or cardiac pathology. Platelets were isolated by centrifugation of plasma that had been isolated from patient’s citrated peripheral blood. Patients’ platelets were used to assess the functional activity of receptors. We used the following agonists involved into physiological regulation of hemostasis: adenosine diphosphate (ADP; 5 µm) which ensures autocrine stimulation of platelets, is accumulated in platelet dense granules, and maintains the platelet proaggregation status; adrenaline (5 µm), whose level increases in stress response following activation of the sympathoadrenal system; angiotensin II (AN-II; 2 µm), whose level increases following activation of the renin-angiotensin system; platelet activation factor (PAF, 150 µm), a paracrine mediator that ensures both platelet stimulation and platelet-white blood cell interaction in inflammation; collagen (2 µg/mL), which reflects the effect of blood collagen levels and/or expression of vascular basement membrane collagen IV on the platelet phase of hemostasis. The agonists were obtained from Sigma (St. Louis, MO) and used in EC50 concentrations to produce 50% ± 5 % of the maximum rate of aggregation in healthy individuals (10 donors). Platelet aggregation was assessed spectrophotometrically with a Chrono-Log aggregometer (Chrono-Log Corp, Havertown, PA). Statistical analysis was performed using Medstat Software. The mean (M) and standard error of the mean (m) were calculated for the two groups. Statistical comparisons between the two groups were performed using a Student's t-test in the case of quantitative, normally distributed variables and a Wilcoxon rank-sum test in the case of quantitative, not normally distributed variables. Differences were considered statistically significant at P < 0.05. Pearson correlations were performed to evaluate the association of normally distributed variables and Spearman's ? correlations when variables exhibited a non-normal distribution. Results Thirty eight patients (mean age, 67.9 ± 1.5 years) were found to have DR in the absence of fundus changes. The majority of patients were aged between 65 and 80 years (24 (63.2 %)); therefore, the development of DR in them took place against the background of aging, which might be associated with the risk of atherosclerotic damage to cerebral arteries. The study group included 11 men (28.9%; mean age, 68.9 ± 2.3 years (95 % CI percentile 61-76 years) and 27 women (71.1 %; mean age, 67.3 ± 1.4 years (95 % CI percentile 63-74 years); age difference between sexes was not significant (p = 0.572). The majority of male patients were aged between 75 and 80 years (5 (45.4 %)), while the majority of female patients were aged between 65 and 75 years (20 (74.1 %)). Further development of diabetic retinopathy is associated with retinal microcirculation impairment due to increased PAG capacity. Therefore, it was required to answer a key question: whether the reactivity of platelets in diabetes patients without fundus changes is different from that in healthy individuals? We found platelet hyperreactivity to all agonists under study in study patients versus controls (Table 1). Patients had platelet responses to collagen, adrenaline, AN-II, ADP, and PAF increased by 56.2 % (р < 0.001), 57.4 %, 32 %, 15.7 % and 21.2 % (р < 0.001), respectively, compared to controls. Therefore, in diabetes patients showing no fundus changes we found platelet responses to humoral agonists which reflect the role of etiopathogenic factors (activation of the sympathoadrenal and renin-and-angiotensin systems, development of inflammation, exposure of vascular basement membrane collagen, increased autocrine platelet stimulation) in the induction of thrombogenesis. 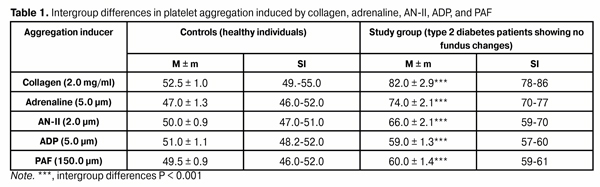 It is noteworthy that it is collagen that is a key inducer of PAG; this can be confirmed by high values of the first and third quartiles of platelet aggregation (77% and 87%, respectively) in our study. Other agonists were found to be substantially less potent inducers of platelet aggregation. Thus, the percentage ratios of platelet responses to adrenalin, AN-II, ADP, and PAF, to collagen-induced PAG (EC50) were 90.2 %, 80.5 %, 72 % and 73.2 %, respectively (p < 0.001). These findings demonstrate an elevated GPVI-mediated response to collagen, i.e., collagen-induced platelet reactivity status; it is important that this status can be verified through in vitro testing. Analysis of platelet responses to various agonists allows us to assess functional integration of various components of intracellular signal systems which enable PAG. When comparing the correlation between adrenaline-induced PAG and PAF-induced PAG (r = 0.734) and the correlation between the former and AN-II-induced PAG r = 0.900 (р < 0.001), one may conclude that the function of the common signaling pathway downstream of Gi and Gq proteins may be effective when ?2-adrenoreceptors, PAF receptors and AT1 receptors are activated. We might hypothesize that proaggregatory activity of platelets increases with the interaction between andrenaline and PAF (and/or AN-II). The presence of statistically significant correlations between AN-II-induced PAG, ADP-induced PAG and PAF-induced PAG (r=0.557, р = 0.03; r = 0.604, р < 0.001; and r = 0.607, p = 0.001, respectively) evidences that AT1 receptors can interact both with purin receptors and PAF receptors in regulation of platelet function. This platelet activation mechanism may occur on a condition of effective functioning of the common signaling pathway of the proper agonist signaling system which is associated with the pathway downstream of Gq protein. Also of importance are statistically significant correlations between, on the one hand, AT-induced ADP and, on the other hand, adrenaline, PAF or collagen (r = 0.643, r = 0.745 and r = 0.617, respectively; р < 0.001). Such an association of proaggregatory effects of agonists may reflect the potential interaction between purine receptors and ?2-adrenoreceptor, PAF receptor and GP VI receptors due to maintenance of the activity of phospholipase C which is a common signal transducer for the action of Gi and Gp proteins and tyrosine kinases of platelet activation signaling system. Therefore, in type 2 diabetes patients showing no fundus changes, platelets were characterized by specific functional activities of GP VI receptors, ?2-adrenoreceptors, АТ1 purine receptors (P2Y12 and P2Y1 receptors) and PAF receptors. Hyperreactivity was a prevailing type of platelet receptor response to agonists. Each type of receptor was characterized by a specific percentage platelet aggregation range related to the greatest share of patients under investigation: collagen, 80-90 %; adrenaline, 75-80 %; AN-II, 70-80 %; ADP and PAF, 60-65 %. If persistent, platelet hyperreactivity to one or more agonists can lead to thrombogenesis and ocular microcirculation abnormalities. Discussion As ocular microcirculatory changes has been associated with thrombogenesis and are accompanied by retinal ischemia and hemorrhages and blood-retinal barrier breakdown [3], platelet studies for the assessment of the degree of abnormalities in microcirculation and of the stage of DR seems promising. Thus, Kubisz and coworkers have proved that platelets are involved in the pathogenesis of diabetic microangiopathy and this is associated with elevated platelet activity [4]. Therefore, predictors of endothelial and platelet disorder could improve the screening of individuals at increased risk, thus leading to the early diagnosis, appropriate treatment, as well as to the effective prevention of the complications of DM2 [4]. Yilmaz and Yilmaz [5] have pointed to the association between morphometrical platelet parameters (mean platelet volume, platelet size, platelet large cell ratio and platelet count) and the stage of DR, and concluded that mean platelet indices could be a beneficial prognostic marker of DR in patients with DM2. This conclusion has been confirmed by Soma and coworkers [6] who revealed a substantial activation of platelets in diabetes patients in their study with markers indicating platelet activation (CD62P-PE and CD63-PE). Correspondingly, the question arises, whether platelets could be a promising model for DR research. First, it has been proved that DM is associated with high reactivity of platelets, with mean platelet volume, platelet distribution width (PDW), platelet–lymphocyte (P/L) ratio being higher in DM2 patients than in control patients, although there was no difference between the DM2 + DR and the DM2 without DR groups [7]. In addition, the study by Miao and co-authors [8] (2016) confirmed that DM2 is associated with platelet hyperreactivity. It seemed expedient to them to investigate if platelet dysfunction might hamper platelet angiogenic activities in DM2 patients. Those authors concluded that platelet activation induced by high doses of PAR1 peptide thrombin receptor agonist (PAR1-AP) results in increased secretion of angiogenic regulators in mild DM2. Therefore, diabetes mellitus is characterized by platelet hyperreactivity, which was further demonstrated in our in vitro study. We find it important to continue searching for informative and specific markers of morphological and functional status of the platelet population which can demonstrate the development of diabetic retinopathy; this requires further research. Second, high individual platelet reactivity was observed in patients of the study, and it explains failures to find informative indices for average intragroup functional activity of platelets for patients with progressing diabetes. This has been pointed to by Kap?on-Cie?licka and collaborators [2] who aimed to evaluate variability of platelet reactivity in patients with DM2 treated with oral antidiabetic drugs and receiving chronic ASA therapy. They found poor agreement between different tests of platelet function. Thus, Aspirin Reaction Units (ARU) ? 550 by VerifyNow® was found in 14.0% of patients, whereas collagen-epinephrine closure time (CEPI-CT) below median of readings other than 300 s (PFA-100®) was found in 32.8% of patients. Therefore, DM2 patients were characterized by large variability of platelet reactivity, with little agreement between various methods. The problem is due to the individual reactivity of the patient, which is reflected in the variations in functional activity of investigated GP VI receptors, ?2-adrenoreceptors, АТ1 purine receptors (P2Y12 and P2Y1 receptors) and PAF receptors among DM2 patients showing no diabetic fundus changes in our study. Conclusion The analysis of functional activity of platelet receptors would provide insight into the clusters of platelet receptors which are capable of maintaining the platelet proaggregation status and causing abnormalities in ocular microcirculation, and thus causing progression of DR. Therefore, studies of platelets in vitro may be promising in the context of analysis of the development and progression of diabetic retinopathy. References
|
