Severe corneal infections induced by contact lens wearing
Drozhzhina G.I., Dr. Sc. (Med.), Prof.
Ivanova O.N., Cand. Sc. (Med.)
Ostashevskii V.L., Cand. Sc. (Med.)
Gaidamaka T.B., Dr. Sc. (Med.)
Ivanovskaia E.V., Cand. Sc. (Med.)
Kogan B.M., Cand. Sc. (Med.)
Usov V.Ia., Dr. Sc. (Med.)
Filatov Institute of Eye Diseases and Tissue Therapy, NAMS of Ukraine
Odessa, Ukraine
Background. Over the recent years, a lot of authors have noticed changes in risk factors of microbial keratitis development as well as in variety of agents inducing them. Soft contact lens wearing has been reported to be the leading risk factor for infectious keratitis.
Purpose. To analyze cases of severe infectious inflammations in the cornea which are associated with contact lens wearing.
Materials and methods. We followed up 60 patients (64 eyes) with severe infectious corneal inflammations aged 15 to 63 y/o (М=35.3±SD20.3) who used contact lenses to correct myopia and hypermetropia. CLs were used to correct mild myopia in 10 patients; moderate myopia in 27 patients; and high myopia (6.5D – 20.0D) in 20 patients. Three patients used CLs to correct mild hypermetropia. There were deep stromal keratitides in 16 eyes; corneal ulcers in 34 eyes (including with stromal melting and corneal perforation, 14 and 6 eyes, respectively); corneal abscess-complicated ulcers in 4 eyes; anterior endophthalmitis in 10 eyes. The process was bilateral in four patients. CLs had been used for 0.5 to 44 years (М=9.41±SD7.33). Prior to admission to the Filatov Institute, the patients had received community-based care for 1 to 75 days (M= 21.6±SD19.64).
Result. Inflammation was managed and the eye as an organ was preserved in 98.5% of cases (63 yes). Evisceration was performed in one eye. Prospects for surgery with optic purpose were preserved in 54 patients (84.3%).
Conclusions. CL users should be dispensary observed as well as be informed of possible infection complications when wearing CL and follow CL using and storage regulations. Once complaints appear it is necessary to attend an ophthalmologist. When infection complications are diagnosed, patients should be immediately referred to a specialized medical institution of a higher level.
Key words: contact lenses, infectious corneal inflammations, treatment
Over the recent years, a lot of authors have noticed changes in risk factors of microbial keratitis development as well as in variety of agents inducing them [33, 6]. Soft contact lens wearing has been reported to be the leading risk factor for infectious keratitis. To date, these keratitides have been marked as a separate group of contact-lens-wear-related keratitides [5, 12, 16, 17, 18]. In 2012, investigations in Wills Eye Hospital showed that infectious keratitis was associated with contact lens wearing in 44% cases of 500 infectious keratitis patients and every other case (46%) referred to severe corneal damage [11].
Multi-centre trial performed in the Netherlands has shown that risk of microbial keratitis development, when wearing soft contact lens, increases 20 times, and 5 times more if lenses are not taken off for a night [7]. Other authors have pointed that complications can develop 60 times more often in contact lens wearers [9, 10].
A retrospective study carried out in Germany from 2005 to 2010 has demonstrated that among 65 patients with keratitis associated with contact lens wear, bacteria have been revealed in 81%; herewith, bacteria revealed are mostly gram-negative including Pseudomonas aeruginosa, Klebsiella oxytoca, Serratia spp. [4]. Pseudomonas aeruginosa is an extremely dangerous infection for the cornea since protease of the former can quickly melt the corneal stroma and be complicated by perforation [14].
Every year, infectious keratitis is diagnosed in one of 2 500 contact lens wearer in the daytime only and in one of 500 continuous contact lens wearers [16, 18].
A new risk group for CL-wear-related infectious keratitis development is children and adolescents who use CLs stabilizing a shape of the cornea to correct myopia and astigmatism. Using orthokeratology lenses, which are worn at night, and in a case of their insufficient placing on the cornea results in epithelium defect occurrence often with bacterial keratitis [10, 11].
Since a number of patients using contact lenses increased up to 80 million people over the last decades and soft contact lenses (SCL) as a means of optical correction for various refractive abnormalities take the leading position, it is expected that a number of patients with CL-wear-associated keratitis would increase [20]. This can be caused by social and cultural, hygienic factors as well as by good adhesive properties of bacteria and protozoa (acanthamoeba) to siliconhydrogel which CL is made of [15]. Besides, it is known that microbes create a biofilm on the surface of CL, not depending on CL type; and this biofilm protects infection agents from action of disinfecting drugs and antibiotics [19].
Data of literature and our observations have shown that consequences of severe corneal inflammations induced by CL wearing are the cause of vision loss, decrease in life quality, and increase in working population disability rates [1, 2, 13]. That’s why, prophylaxis of infectious inflammations, their timely diagnosis and early and successful treatment is a current issue in ophthalmology
Thus, the purpose of the present study was to analyze cases of severe infectious inflammations in the cornea which are associated with contact lens wearing.
Materials and methods
60 patients (64 eyes) with severe infectious corneal inflammations associated with contact lens wearing to correct refractive errors underwent inpatient treatment at Corneal Pathology Department at the Filatov Institute from 2010 to 2015. Four patients had bilateral process. All the patients were of working age from 15 to 63 y/o (М=35.3±SD20.3). CLs had been used for 0.5 to 44 years (М=9.41±SD7.33).
CLs were used to correct mild myopia in 10 patients, moderate myopia in 27 patients, and high myopia (6.5D – 20.0D) in 20 patients. Three patients used CLs to correct mild hypermetropia.
58 patients used soft contact lenses, mainly, with scheduled replacement at 1, 3, 6 months and flexible and prolonged wearing schedule. Two patients used orthokeratology lenses.
Prior to admission to the Filatov Institute, the patients had received community-based care for 1 to 75 days (M= 21.6±SD19.64).
Results
Clinical forms of corneal damage were as follows: deep stromal keratitides, 16 eyes; corneal ulcers, 34 eyes (including with stromal melting and corneal perforation, 14 and 6 eyes, respectively); corneal abscess-complicated ulcers, 4 eyes; anterior endophthalmitis, 10 eyes.
Biomicroscopy of the cornea showed that the area of inflammation varied from 0.5 mm to 11 mm (M=6.21± SD2.29). Monofocal inflammation was noted in 55 eyes while multifocal infiltrates were in 9 cases. Inflammation was superficial in 5 cases (7.81%). In most cases, 59 eyes (92.2%), was noted deep-lying inflammation. Inflammation 1/3 and 2/3 of corneal stroma thickness deep was recorded in 21 cases (32.8%) and 8 cases (12.5%), respectively; full thickness infiltrates were observed in 30 cases (46.9%).
The presence of hypopyon 0.5 to 4 mm high was noted in 11 cases (17.1%), fibrinous exudates in anterior chamber and in the pupil site were in 12 eyes (18.7%) and 2 eyes (3.1%), respectively.
Inflammation location was central, paracentral, and peripheral in 42 (65.6%), 18 (28.1%), and 4 (6.25%) cases, respectively. Imaging of anterior chamber structures was possible to perform in 43 cases (67.1%) while that was troubled in 21 (32.8%) cases. Secondary hypertension and ocular hypotony were noted in 29 (45.3%) and 7 (21.8) cases, respectively.
Historical study showed that in all cases patients had not practice proper contact-lens-wear hygiene as wearing schedule and lens treatment and storage regulations.
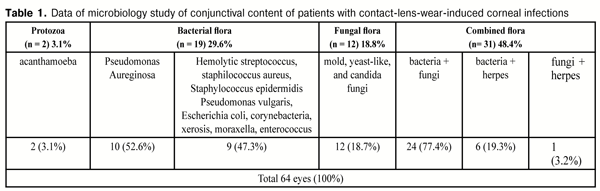
Microbiology study of conjunctival content from the infected eye revealed bacterial flora in 19 (21) eyes (29.6%), fungal in 12 (18.8%), combined in 31 (48.4%), and protozoa in 2 eyes (3.1%) (Table 1). Among bacterial flora prevailed Pseudomonas aureginosa, 10 cases (52.6%). In rest of cases, there were different types of staphylococcus, Escherichia coli, moraxella, enterococcus. Fungal flora included mold, yeast-like, and candida fungi. In case of a combined flora, bacterial and fungal infection prevailed over others (77.4%). Of protozoa was detected acanthamoeba (3.1%).
Each patient underwent intensive etiotropic and pathogenetic treatment which included local and systemic antibacterial, antiprotozoal, and anti-fungal drugs as well as non-steroid anti-inflammatory, desensitizing, and detoxification drugs.
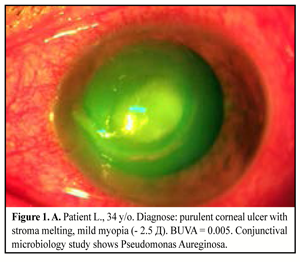
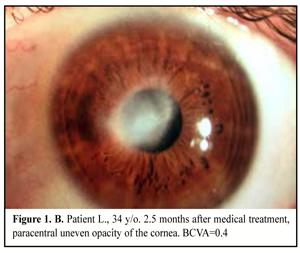
34 patients (38 eyes) were performed medical treatment with forced schemes of antibacterial and antifungal drugs. Local absorbents and dry sulfacetamide powder were used in patients with corneal abscess; mydriatics were used to prevent from synechiae; in two cases photodynamic therapy with methylene blue was added (Figure 1. A, B).
A forced scheme of antibacterial drugs included a combination of two groups of broad-spectrum antibiotics with different action mechanisms: aminoglycosides (gentamicin) and third and fourth generation fluoroquinolones – moxifloxacin, levofloxacin (Levoximed, Oftaquix) every hour during the first two days, and, afterwards, every two hours during the following 3-5 days. Investigations performed by Kramer A. as well as by Rachwalik D. and Pleyer U. have shown that conjunctival microflora is most sensitive to moxifloxacin (92%) and gentamicin (89%) in comparisom with other antibacterial drugs [8, 11]. The further prescription of antibacterial drugs was corrected in accordance with microbiology test results. Systemic patients received on medical indications third generation cephalosporin (ceftriaxone), third and fourth generation fluoroquinolones (levofloxacin, gatifloxacin), aminoglycosides (gentamicin, amikacin), imidazole derivative (metronidazole, ornidazole), or combined levofloxacin and ornidazole drug.
If fungal infection occurred, antiseptic solutions were installed locally including 0.02% chlorhexidine, 2.0% potassium iodide 4-6 times a day as well as 0.2% fluconazole, 015-025% amphotericin b 6-8 times a day. Systemic fungal inflammation patients received fluconazole No15-20 by i.v. infusion or per os during 4-6 weeks, or itraconasole, ketoconazole according to the schedule.
In the presence of allergic or toxic and allergic reactions resulted in long-lasting uncontrolled using antibacterial drugs, treatment included olopatadine derivates (Pallada) twice a day or cromoglicic acid (Allergocrome, Lecrolyn) four times a day as well as desensitizing drugs orally to inhibit histamine receptors and to stabilize mast cells.
Patients with secondary hypertension received locally non-cardioselective ?1- and ?2-beta-adrenoceptor blockers (Arutimol 0.5%, Timolol 0.5%), selective alpha-2-adrenergic agonists: brimonidine, carbonic anhydrase inhibitors (Dorzamed) two times a day as well as combined drugs (beta-blocker + carbonic anhydrase inhibitor two times a day. Intake, diacarb was prescribed 1 tab. twice a day against the background of potassium preparation intake; osmotic diuretics (Lasix i/m, Mannit i/v) were prescribed in severe cases.
When medical treatment was not successful, i.e. inflammation expanded in area or in stromal depth, corneal tissue was lyzed and thinned, there was a risk or in the presence of perforation, and inflammation was spread onto limbus and sclera area, therapeutic keratoplasty was performed. The purpose of the surgery was: to manage inflammation, to resorb inflammatory infiltration, to recover anatomic integrity of the cornea, and to preserve the eye as an organ (Figure 2. A, B, C).
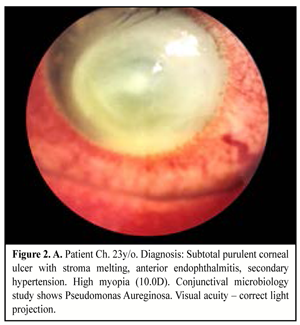
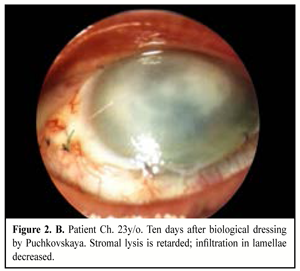
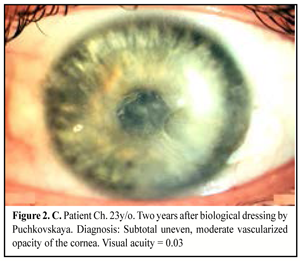
Keratoplasty (KP) with therapeutic purpose was performed in 25 eyes, of them penetrating KP in nine eyes (36%) including step penetrating keratoplasty in five eyes; lamellar KP in eight eyes (32%); biological dressing by Puchkovskaya in eight cases (32%).
When performing therapeutic penetrating KP, we removed from the anterior chamber exudates, retrocorneal and intrapupillar films, separated anterior and posterior synechiae, released the anterior chamber angle, performed basal iridectomy, and washed the anterior chamber by antibiotic and antifungal medications as indicated.
In the presence of pseudomonas inflammations which are accompanied with rapid lysis of the corneal tissue, we performed urgent biological dressing by Puchkovskaia in eight eyes to avoid corneal perforation.
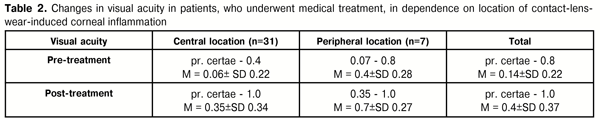
In the group of patients who underwent medical treatment, VA increased in 86% of cases. Before treatment, VA in patients with central location of inflammation (31 eyes) was correct light projection to 0.4 and averaged 0.06 (SD±0.22). After therapy performed, VA in this group increased at average to 0.35 (SD±0.34), with maximal increase to 1.0. In patients with peripheral location of inflammation (7 eyes), VA was 0.07 to 0.8 (average 0.4 (SD±0.28)) before treatment and increased to 0.7(SD±0.27) with 1.0 as maximum (Table 2).
Penetrating KP performance resulted in transparent, semi-transparent, and opaque implant survival in 1, 5, and 3 cases, respectively. As a result of lamellar KP, transparent, semi-transparent, and opaque implant survival was observed in 5, 2, and 1 case, respectively.
In a result of biological dressing we managed to retard inflammation progression and destruction of the corneal tissues. Postoperatively, the patients received etiotropic pathogenic and symptomatic therapy. On completion of corneal surface epithelialization, a combined drug Neladex was used locally four times a day for 2-3 weeks; Neladex contains dexamethasone and two antibiotics with a wide spectrum: neomycin (aminoglycoside) and polymyxin B. The former has a bactericidal action, disturbs protein synthesis in microbial cells and is active in relation to gram-positive and gram-negative microorganism. The latter is an antibiotic of polypeptide structure; the mechanism of its action is conditioned with ability to be connected with microbial cell membrane phospholipids that leads to their destruction. Polymyxin B is active mainly in relation to gram-negative flora Pseudomonas aeruginosa.
Apart from the combined drugs, dexamethasone (Medexol, Dexapos) was instilled 4-5 times a day according to decreasing medication regimen for 4-6 months. To accelerate the epithelium regeneration, we used dexpanthenol as well as preservative-free hyaluronic acid-based tear substitutes or hyaluronic acid with dexpanthenol (Hylo-care) 4 times a day.
Secondary hypertension in medically treated patients was recorded in 13 cases (34.2%). Herein, it was compensated in all cases.
Glaucoma was observed in 16 cases (61.5%) in group of therapeutic KP treated patients. Of them, intraocular pressure was compensated in 11 cases; glaucoma surgery was performed in 5 patients (31%).
As a result of treatment performed, inflammation was managed in 98.5% of cases (63 eyes). Evisceration was performed in one eye. Prospects for surgery with optic purpose were preserved in 54 patients (84.3%). Thus, early intensive adequate treatment using therapeutic KP enables, in most cases, to manage inflammation, to preserve the eye as an organ and the prospects for further surgery with the optic purpose.
Conclusions
1. Infectious corneal inflammations associated with SCL wearing are especially severe (Pseudomonas aeruginosa was infection agent in 52.6%).
2. CL users should be dispensary observed as well as be informed of possible infectious complications when wearing CL and follow CL using and storage regulations. Once complaints appear it is necessary to attend an ophthalmologist.
3. When infection complications are diagnosed, patients should be immediately referred to a specialized medical institution of a higher level.
References
1. Egorova GB. Optimization of contact correction of primary and secondary ametropy. Thesis for Dr. Sc. (Med.). M.; 2005. Russian.
2. Kivaiev AA, Shapiro EI. Contact correction of the vision. M.; 2000. Russian.
3. Amescua G, Miller D, Alfonso EC. What is causing the corneal ulcer? Management strategies for unresponsive corneal ulceration. Eye (Lond). 2012;26:228-236.
Crossref Pubmed
4. Bohm MR, Prokosch V, Merte RL et al. Mikrobiologishe analyse in kontaktlinsen – assoziierte keratits. Klin Monatsbl Augenheilkd. 2011;228:808-814.
Crossref Pubmed
5. Carnt N, Keay L, Naduvilath T, Holden BA, Willcox MDP. Risk factors associated with corneal inflammation in contact lens wear. Invest Ophthalmol Vis Sci. 2007;48:E-Abstract 4326.
6. Cavanagh HD, Robertson DM, Petroll WM, Jester JV. Castroviejo Lecture 2009: 40 years in search of the perfect contact lens. Cornea. 2010;29(10):1075-85.
Crossref Pubmed
7. Cheng KH, Leung SL, Hoekman HW et al. Incidence of contact-lens-associated microbial keratitis and its related morbidity. Lancet. 1999;354(9174):181-5.
8. Kramer A, Seitz B, Messmer EM. Infektiose keratitis – keimspektrum im wandel der zeit und aktuelle therapien. Ophthalmologe. DOI 10.1007/s00347-016-0324-7/ © Springer-Verlag Berlin Heidelberg. 2016 DOG 2016. Berlin.
9. Morgan PB, Efron N, Hill EA, Raynor MK, Whiting MA, Tullo AB. Incidence of keratitis of varying severity among contact lens wearers. Br J Ophthalmol. 2005;89(4):430-6.
10. Morgan PB. Contact lens compliance and reducing the risk of keratitis. Optician. 2007; 234:20-5.
11. Rachwalik D, Pleyer U. Bacterial Keratitis. Klin Monatsbl Augenheilkd. 2015;232:738-744.
12. Radford CF, Minassian DC, Dart JK. Disposable contact lens use as a risk factor for microbial keratitis. Br J Ophthalmol. 1998;82(11):1272-5.
13. Robertson DM, Cavanagh HD. The Clinical and Cellular Basis of Contact Lens-related Corneal Infections: A Review. Clin Ophthalmol. 2008;2(4):907-17.
14. Robertson DM, Petroll WM, Jester JV et al. The role of contact lens type, oxygen transmission, and care-related solutions in mediating epithelial homeostasis and pseudomonas binding to corneal cells: an overview. Eye Contact Lens. 2007;33: 394–8.
15. Robertson DM. The Effects of Silicone Hydrogel Lens Wear on the Corneal Epithelium and Risk for Microbial Keratitis. Eye Contact Lens. 2013, January; 39(1):67–72.
16. Stapleton F, Keay L, Jalbert I, Cole N. The epidemiology of contact lens related infiltrates. Optom Vis Sci. 2007;84(4):257-72.
17. Szczotka-Flynn L, Pearlman E, Ghannoum M. Microbial Contamination of Contact Lenses, Lens Care Solutions, and Their Accessories: A Literature Review. Eye Contact Lens. 2010;36(2):116–29.
18. Tariq F, Koay P. The Risk of Contact Lens Wear and the Avoidance of Complications. Int J Med Students. 2013;1(2):80-5.
19. Willcox M. Microbial adhesion to silicon hydrogel lenses: a review. Eye Contact Lenses. 2013;39:61-6.
20. Yildiz E, Airiani S, Hammersmith K et al. Trends in contact lens – related corneal ulcer at a tertiary referral center. Cornea. 2012;31:1097-102.







