J.ophthalmol.(Ukraine).2016;5:44-46.
|
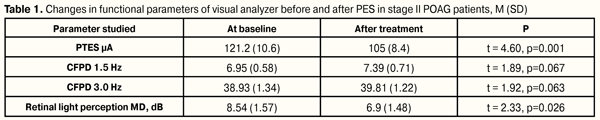
https://doi.org/10.31288/oftalmolzh201654446 Results of phosphene electrostimulation treatment of patients with primary open-angle glaucoma V. A. Putienko V.S. Ponomarchuk, Dr Sc (Med), Prof. Filatov Institute of Eye Diseases and Tissue Therapy, NAMS of Ukraine Odessa, Ukraine E-mail: alputienko@ya.ru The purpose was to assess the efficacy of phosphene electrostimulation in patients with stage II and III of primary open-angle glaucoma with compensated IOP. Material and Methods. We followed up 26 patients (31 eyes) with drug-compensated primary open-angle glaucoma (POAG). 16 eyes with stage II glaucoma comprised the first group. The second group consisted of 15 eyes with stage III glaucoma. Treatment included 10 PES standard sessions. Each session lasted 10 minutes. Current dose was selected in every particular case in regard to initial зhosphene threshold of electric sensitivity (PTES) level. Treatment efficacy was assessed according to rate changes in PTES, CFPD in «1.5» and «3» modes and according to changes in computer perimetry data. Results. FES in stage II POAG patients enabled to improve retinal light perception by 19.3% and to increase electrical sensitivity of visual analyzer (phosphene threshold increased by 14.4%). In stage III patients, light perception increased by 14.8%, phosphene threshold of electrical sensitivity increased by 15.2%, and phosphene electrical lability in modes 1.5 and 3.0 increased by 13.5% and 21.1%, respectively, which made it possible to recommend the treatment for this category of patients. Key words: primary open-angle glaucoma, phosphene electrical stimulation, phosphene threshold, critical frequency of phosphene disappearance Introduction Primary open-angle glaucoma has a special place in the structure of eye diseases and invalidity. According to data of World Health Organization, about 600 000 glaucoma-associated blindness cases are newly registered every year. A total of glaucoma patients worldwide has exceeded 100 000 000 people, of them 10.8 million are blind in both eyes [1]. Though much research has been done and a wide range of modern diagnostics is available, pathogenesis is still not fully understood. The question on structural damage primacy in glaucoma neuropathy remains open. A number of researches have put the death of retinal ganglion cells (RGC) on the first place while others traditionally name injury of RGC axons at the level of lamina cribrosa of the optic disc (OD) [9]. Glaucoma neuroretinopathy can develop in the retina and optic disc at the same time but in different pathogenic ways [6] with prevalence of mechanic or vascular factors [2, 4]. Thereby, ischemic alterations lead to axoplasmatic current blockage and neurotrophic deficiency, ATP reduction, glutamate elevation, NMDA receptor activation, excessive entry of calcium ions into a cell, DNA fragmentation, and, as a consequence, to apoptosis of retinal ganglion cells. This pathophysiological mechanism is accompanied by chronic oxidative stress-associated inflammation, matrix metalloproteinase expression, increased pro-inflammatory cytokine production and genetic factor activation [6]. Modern neuroprotective medication-based methods of treatment for glaucoma neuropathy are not successful enough, which requires development of new approaches to treatment [4]. One of the treatments for optic nerve diseases is phosphene electrical stimulation (PES) Electric stimulation is known to intensify transport and metabolic processes in axons of glial and tissue connective elements as well as to accelerate the renewal of cellular membrane phospholipids with increased DNA synthesis. PES improves the activity of visual analyzer since the eye and cerebral visual centers are better supplied with blood [3, 5, 7]. In the literature there is a single research on PES treatment in glaucoma patients [8] that is used as a reason to conduct the present investigation. The purpose was to assess the efficacy of PES in patients with stage II and III of primary open-angle glaucoma with compensated IOP. Material and Methods We followed up 26 patients (31 eyes) with drug-compensated primary open-angle glaucoma. Glaucoma was diagnosed according to the data of ophthalmoscopy, gonioscopy, tonography, computer static perimetry and optical coherence tomography. 16 eyes with stage II glaucoma comprised the first group. The second group consisted of 15 eyes with stage III glaucoma. The age of patients in the first and second groups averaged 69 SD (4.97) and 70 SD (4.79) y/o, respectively. Mean vision acuity in patients with glaucoma of stages II and III equaled 0.78 SD (0.13) and 0.34 SD (0.13), respectively. Mean IOP value by Makhlakov was 16.56 SD (1.0) mm and 17.1 SD (1.1) mm in the first and second groups, respectively. Optical coherence tomography (OCT) was performed using Carl Zeiss (CIRRUS Photo 800). We took into account the mean thickness of retinal nerve fiber layer (RNFL) which equaled 68.6 SD (2.9) mm and 55.7 SD (4.5) mm, in the first and second groups, respectively; furthermore, excavation of the optic disc was 0.76 SD (0.037) and 0.87 SD (0.029) in the first and second groups, respectively. Phosphene threshold of electric sensitivity (PTES) and critical frequency of phosphene disappearance (CFPD) were determined using 1.5 and 3.0 modes, and “FOSFEN-1” unit was used to perform treatment sessions. Computer perimetry was performed using OCULUS vision field analyzer with Threshold, 30-2 mode. Treatment included 10 PES standard sessions. Each session lasted 10 minutes. Current dose was selected in every particular case in regard to initial PTES level. Treatment efficacy was assessed according to rate changes in PTES, CFPD in 1.5 and 3.0 modes and according to changes in computer perimetry data. Data are given as a mean and standard deviation in brackets M (SD). Statistical processing was performed with STATISTICA 7.0 software. Analysis was performed using pairwise Student t test and nonparametric ?2 criterion. Critical p-level was equal to 0.05. Results In the eyes with stage II glaucoma, PTES was 121.2 (10.6) µA at baseline and significantly decreased after treatment to 105 (8.4) µA, or by 14.4% (р = 0.001). CFPD parameters did not change significantly in both 1.5 and 3.0 modes: from 6.9 (0.6) Hz to 7.4 (0.7) Hz (р = 0.067) and from 38.9 (1.34) Hz to 39.8 (1.2) Hz (р = 0.068), respectively. Herewith, significant improvement was noted in static computer perimetry parameters: mean deviation (MD) of retinal light perception decreased from 8.5 (1.6) dB to 6.9 (1.5) dB, or by 19.3% (р = 0.026) (Table 1)
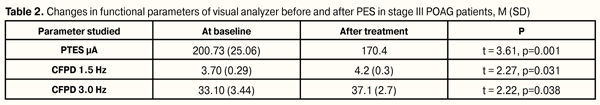
Therapy performed did not influence significantly on IOP in stage II POAG patients. Mean post-treatment IOP was equal to 16.88 (0.71) mm vs. 16.56 (1.09) mm (t = 1.77, р=0.071) at baseline. Also, no significant difference was noted in visual acuity (VA) before and after treatment (t = 1.61, р=0.093). Mean VA before and after treatment equaled 0.77 (0.14) and 0.80 (0.13), respectively. In the eyes with stage III primary open-angle glaucoma, PTES decreased significantly from 200.7 (25.1) µA to 170.4 (20.8) µA, by 15.2% (р = 0.001). CFPD parameters were increased significantly at both 1.5 and 3.0 modes, from 3.7 (0.3) Hz to 4.2 (0.3) Hz or by 13.5% (р = 0.031) and from 33.1 (3.44) Hz to 37.1 (2.7) Hz or by 12.1% (р = 0.038), respectively. Like in stage II POAG patients, in this group we noted significant improvement in static computer perimetry parameters: mean deviation (MD) of retinal light perception decreased from 13.7 (0.7) dB to 11.7 (0.9) dB or by 14.8% (р = 0.001) (Table 2).
Like in group of patients with stage II POAG, therapy performed did not influence significantly on IOP in stage III POAG patients. Mean post-treatment IOP was equal to 17.33 (0.12) mm vs. 17.13 (0.9) mm (t = 1.38, р=0.019) at baseline. Also, no significant difference was noted in (VA) before and after treatment (t = 1.87, р=0.081). Mean VA before and after treatment equaled 0.33 (0.11) and 0.35 (0.14), respectively. Conclusion Progression of the optic nerve neuropathy is the main cause of visual function reduction in POAG patients. Medicated neuroprotection applied in patients of this category is not very successful at the present time and requires the further improvement. PES treatment was proved to be successful for partial optic nerve atrophy of different geneses [3]. In the literature, there is a single report on the use of this treatment in POAG patients [8]. The authors have noted the beneficial effect of the therapy. In the present paper, we assessed the effect of PES in POAG patients with different stages: stage II, when optic nerve damage degree was less, which was confirmed to OCD data; and stage III, when according to OCT data significant nerve fiber thinning occurred and pronounced optic disc excavation comparing to norm was noted. The research performed showed that PES was more successful for stage III POAG patients, that was expressed in the improvement of functional activity of optic nerve axial area, in both 1.5 and 3.0 modes. Thus, we can conclude that the use of PES in stage II POAG patients enabled to improve retinal light perception by 19.3% and to increase electrical sensitivity of visual analyzer (phosphene threshold increased by 14.4%). In stage III patients, light perception increased by 14.8%, phosphene threshold of electrical sensitivity increased by 15.2%, and phosphene electrical lability in modes 1.5 and 3.0 increased by 13.5% and 21.1%, respectively, which made it possible to recommend the treatment for this category of patients.
References 1.Alexeev VN, Levko MA, Al–Gifari AM. [Comparison of effects of Xalatan, Travatan and Tafluprost usage in treatment of primary glaucoma]. Klinicheskaia Oftalmologiia. 2008;9(3):108-10. Russian. 2.Bobr TV, Rozhko JI. [Functional activity of the retina in compensated glaucoma]. ARSmedica. 2011;16(52):52–5. Russian. 3.Drozhenko V.S. The influence of the modificate phosphenelectrical stimulation on the visual functions of patients with partial optic nerve atrophy. Thesis for a candidate’s degree. Odessa; 2002. 158 p. Russian 4.Zavgorodniaia NG, Pasechnikova NV. [Primary glaucoma: A new look at an old problem]. Zaporizhzhia: Orbita-YUG; 2010. p. 192. Russian 5.Ponomarchuk VS, Degtyarenko TV, Chaura AG et al. [Mechanisms responsible for the curative effect of phosphen-electrostimulation]. Neurophysiology. 1998;30:431. doi:10.1007/BF03027698 6.Strakhov VV, Alekseev VV. [The pathophysiology of a primary glaucoma — "all or anything"]. Glaucoma.2009;2:10-2. Russian. 7.Chaura AG. [Physiological mechanism of realization of visual analyzer electrical stimulation effect]. Thesis for Cand. Of Sc. (Biol.) Odessa; 2009. 170 p. Ukrainian. 8.Shevchenko ON, Yusupov RG, Muldashev ER. [The effectiveness of the combined use of transcutaneous electrical stimulation and combination therapy in the treatment of primary compensated glaucoma]. Proceedings of All-Russian scientific practical conference “Glaucoma Millennium: Results and Prospects”. Moscow; 1999. 336-8. Russian. 9.Kerrigan Baumrind L, Quigley H, Pease M et al. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Invest Ophthalmol Vis Sci. 2000;41:741–8.
|