J.ophthalmol.(Ukraine).2016;4:38-42.
|
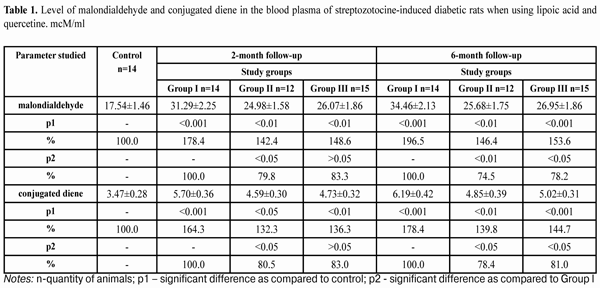
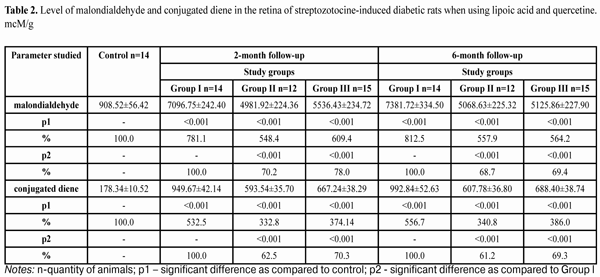
https://doi.org/10.31288/oftalmolzh201643842 Study on the effect of quercetine and lipoate on lipid peroxidation in the retina in experimental diabetes N.Pasyechnikova, Corr. Member of NAMS of Ukraine, Prof. 1 O.Moroz, Cand Sc (Med)2 1 SI "Filatov Institute of Eye Diseases and Tissue Therapy of NAMS of Ukraine "(Odessa, Ukraine), 2 A. Nowak Transcarpathian Regional Clinical Hospital, Ophthalmology department (Uzhgorod, Ukraine) E-mail: moroz.oleg@gmail.com The experiment was performed on white rats and content of malondialdehyde and conjugated dienes in the retina and blood plasma in the condition of streptozotocine-induced diabetes development and quercetine and lipoate application was studied. In such conditions, the use of drugs studied significantly prevented an increase in temporary products of lipid peroxidation (LPO) in the retina and blood of streptozotocine-induced diabetic animals at all terms of diabetes development. The use of lipoic acid in a greater degree decreased the enrichment of final products of LPO in the retina and blood of streptozotocine-induced diabetic animals at all terms of diabetes development as compared to that when quercetine was used. Key words: streptozotocine-induced diabetes, retina, malondialdehyde, conjugated diene, experiment Introduction Blood vessel diseases of the eye, in particular diabetic retinopathy (DR), are the leading cause of vision loss and blindness in the developed countries [1,2]. There are no adequate effective methods to prevent and treat DR for now, taking into account the complicacy and diversity of pathogenesis of the disease causing metabolic desorders and deep structural and functional changes in the retina [3,4,5,6,2]. Many papers on molecular machanisms of DR development have laid the groundwork for a targeted search of pathogenetic-oriented methods of DR prevention and treatment [3,7,8,6,9]. Recent researches have evidenced the role of free-radical processes, in particular lipid peroxidation (LPO) in diabetic-related blood vessel disorders in the retina and other organs. In this regard, of immediate interest are researches on retinal pigment epithelium which state under DR condition is extremely understudied [10,11,12,13,14,15,16]. Oxidative stress which is accompanied by significant increase in level of free radicals and leading to lipid perioxidation increase (conjugated diene, malondialdehyde etc.) blocks protein and nucleic acid synthesis, supresses glycolysis, and enables decoupling of oxidative phosphorylation, inhibits the activity of certain enzymes (glucose 6-phosphatase, adenylate cyclase and others) that leads to dysfunction of many tissues. Superoxide radicals, in particular, activate lipid peroxidation (LPO) [12,13,9,14,15,17]. Experimental studies have determined that under the conditions of modelled streptozotocine-induced diabetes, a significant increase of LPO products in the blood and retina is noted as early as 28 days, herewith at the latter case there is a sharp rise in the concentration of malondialdehyde [18]. It has been found that using vitamers В6 and thiamine derivatives (Benfotiamine, Milgamma) significantly decreases accumulation of LPO end products (malondialdehyde and conjugated diene) in the retina of streptozotocine-induced diabetic rats at all time points of follow-up [19,20]. The purpose of the present paper was to study the content of LPO products in white rats with streptozotocine-induced diabetes when using quercetine and lipoate under conditions of relatively long development (6-month follow-up). Material and Methods The experiment was performed at Biochemestry Laboratory of Filatov Eye Disease and Tissue Therapy Institute and involved Whistar rats, weighted 190-210 g. Experimental animals were divided into four groups at every experimental stage (2-month and 6-month diabetes development): control group,14 rats; study group I, diabetic rats receiving no medication, 14 rats (DM-only group); study group II, diabetic rats receiving lipoic acid (LA), 14 rats (DM+LA group); study group III, diabetic rats receiving quercetine (Q), 15 rats (DM+Q group). At each experimental stage, animals with developing diabetes were receiving orally lipoic acid and quercetine during the whole follow-up period. Diabetes was induced by a single streptozotocine intraperitoneal injection (55 mg per 1 kg body weight [5]. The experiment followed the recommendations on research involving animals accepted by International Society for Eye Research. Upon two months from the diabetes development, a part of experimental rats as well those of controls were decapitated with prior sodium thiopental anesthesia (50mg/kg). Upon six months from the diabetes development, the rest of the animals were also sacrificed in accordance to the rules of hangling experimental animals. Immidiately after decapitation, eyes were enucleated and retinas were removed on the ice at the temperature of 0 – 5?С. Homogenate of the retina was prepared in the 1:10 ratio (tissue’s weight: media volume for homogenization). After centrifuging, the content of malondialdehyde and conjugated diene was determined spectrofluorimetrically in the supernatants of the retinas and blood plazma using microcuvettes. The method to determine malondialdehyde content is based on the fact that at the temperature of 100?С in the acid medium, malondialdehyde reacts with 2-thiobarbituric acid, forming a stained trimethine complex with absorption maximum at a wavelength of 532 nm. 0.1 ml of the fluid studied, considering biological material dilution, was added 3 ml of 1% orthophosphoric acid (рН 2.0), 1 ml of 0.6% thiobarbituric acid solution, and 0.1 ml of 0.28% ferrous sulphate solution. Tubes were placed in the boiling water bath for 60 minutes. Afterwards, the tubes were cooled down in the cold water at temperature 0 - 2? С and 4 ml of butanol was added with following well-stirring and centrifuging for 10 minutes with 3 000 rotations per minute. The optical density of the upper phase was measured using a Specol – 210 spectrocolorimeter at a wavelength of 535 nm against butanol. Content calculation for products reacting with thiobarbituric acid was performed due to a malondialdehyde molar extinction coefficient (1.56•105 mole -1•sm-1) and expressed in mcM/ml of blood plasma and in mcM/g of tissue. The coefficient of variation was 5.2 % [21]. The method to determine conjugated dienes is based on the fact that a system of conjugated double bonds appears in polyunsaturated higher fatty acid molecules in peroxidation at the stage of free radical formation; this is accompanied with appearance of new absorption maximum at 233 nm. 0.5 ml of the fluid studied, considering biological material dilution, was added 4.5 ml of extraction mixture of heptane and isopropil alcohol in 1:1 proportion (V:V). After extraction, the mixture was added 0.5 ml of distilled water; 0.5 ml of the upper (heptane) phase of the stratified sample was collected and mixed with 2.5 ml of ethyl alcohol. The optical density was measured using a SF-26 spectrocolorimeter at 233 nm against ethyl alcohol. Content calculation for determining conjugated was performed due to a molar extinction coefficient (2.2•105 mole -1•sm-1) and expressed in mcM/ml of blood plasma and in mcM/g of tissue [22, 23]. The data obtained were statistically processed using SPSS 11.0 package [24]. Results and Discussion Table 1 demonstrates the data on malondialdehyde and conjugated diene content in the blood plasma of white rats with streptozotocine-induced diabetic rats at time points of 2 and 6 months when using lipoic acid and quercetine. As it can be seen in the table, at two months, the level of malondialdehyde equalled (31.29±2.25) mcM/ml and was increased by 78.4% in blood plazma of animals receiving no medication as compared to that of controls (17.54±1.46) mcM/ml; at six months that increased by 96.5% equaling (34.46±2.13) mcM/ml as compared to controls (р<0.001). In group II animals under conditions of lipoic acid using, at two months after baseline, the level of malondialdehyde in blood plazma decreased to (24.98±1.58) mcM/ml which was lower by 20.2% as compared to group I (р<0.05), and at 6 months it equalled (25.68±1.75) mcM/ml which was lower by 25.5% as compared to group I (р<0.01) When using quercetine in group III animals, the level of malondialdehyde in blood plazma was equal to (26.07±1.86) mcM/ml and (26.95±1.86) mcM/ml at 2 and 6 months after baseline, respectively, that was by 16.7% and 78.2% lower than that in group I animals, not receiving the medications (р<0.05). The level of conjugated dienes in the blood plazma of group I animals was equal to (5.70±0.36) mcM/ml and (6.19±0.42) mcM/ml at 2 and 6 months after diabetes development, respectively, which was by higher by 64.3% and 78.4%, respectively, as compared to control (3.47±0.28) mcM/ml (р<0.001). When using lipoic acid in group II animals, the level of conjugated dienes in blood plazma decreased to (4.59±0.30) mcM/ml and (4.85±0.39) mcM/ml at 2 and 6 months after baseline, respectively, i.g. by 80.5% and 21.6% lower as compared to group I animals, not receiving the medications (р<0.05). Under the effect of quercetine, the level of conjugated dienes in blood plazma decreased by 17.0% and 19.0% at 2 and 6 months (р<0,05), respectively, comparing to diabetic animals receiving no medication, and was equal to (4.73±0.32) mcM/ml and (5.02±0.31) mcM/ml, respectively. Table 2 presents the data on the content of malondialdehyde and conjugated diene in the retina of white rats with streptozotocine-induced diabetes at 2 and 6 months when using lipoid acid and quercetine. As it can be seen in the table 2, at two months, the level of malondialdehyde in the retina of group I diabetic animals increased by 781.1% as compared to control and equaled (7096.75±242.40) mcM/g vs. (908.52±56.42) mcM/g of control; at six months it increased by 512.5% as compared to controls, equaling (7381.72±334.50) mcM/g (р<0.001). In group II animals under conditions of lipoic acid using, the level of malondialdehyde in the retina decreased to (4981.92±224.36) mcM/g and (5068.63±225.32) mcM/g at 2 and 6 months after baseline, respectively, or by 29.8% and by 31.3%, respectively (р<0.001), When using quercetine in group III animals, the level of malondialdehyde in the retina was equal to (5536.43±234.72) mcM/g and (5125.86±227.90) mcM/ml at 2 and 6 months after baseline, respectively, that was by 22% and 30.6% lower than that in group I animals, not receiving the medications (р<0.05). The level of conjugated dienes in the retina of group I animals was equal to (949.67±42.14) mcM/g and (992.84±52.63) mcM/g at 2 and 6 months after diabetes development, respectively, which was higher by 532.5% and 556.7%, respectively, as compared to (178.34±10.52) mcM/ml of the controls (р<0.001). When using lipoic acid in group II animals, the level of conjugated dienes in the retina decreased to (593.54±35.70) mcM/g and (607.78±36.80) mcM/g at 2 and 6 months after baseline, respectively, i.g. by 62.5% and 61.2% lower as compared to group I animals, not receiving the medications (р<0.001). Under the effect of quercetine in group III animals, the level of conjugated dienes in the retina decreased by 29.7% and 30.7% at 2 and 6 months (р<0.001), respectively, comparing to diabetic animals receiving no medication, and was equal to (667.24±38.29) mcM/g and (688.40±38.74) mcM/g, respectively. Such a high sensitivity of lipids in the rat’s retina to free radical oxidation revealed can be explained, first of all, by the fact that it contains significant amount polyunsaturated fatty acids. Moreover, the retina is constantly exposed by combined influence of light and oxygen, which also facilitate the accelerated generating of free radicals [10]. Assessing the results of the present paper, the role of pigment epithelium in the protection of the retinal neuroepithelium from oxidative stress should be noted. However, if the high level of free radical generating occurs, the pigment epithelium cells can be also damaged up to apoptosis develops [11]. The increase of LPO products in the blood and especially in the retina tissues observed under the conditions of streptozotocin-induced diabetes suggests that free-radical oxidation of desaturated fatty acids is an important part in the damage of membrane structures of the retina in experimental diabetes. Conclusions 1.6-month streptozotocine-induced diabetes development led to sharp intensification of LPO in the retina and blood of the experimental animals. This is evidenced, first of all, by more than twice increase in the level of malondialdehyde in the blood and multiple (an average of 8 times) increase of this end LPO product in the retina. 2.In the conditions of modelled streptozotocine diabetes, quercetine and lipoic acid facilitated the decrease in the level of LPO intermidiates in the retina and blood plasma of the rats during the whole follow-up period. 3.At all time points, lipoic acid decreased the accumulation of end LPO products in the retina and blood plasma of streptozotocine-induced diabetic rats in a greater degree as compared to quertecine.
References 1.Dedov II, Shestakova MV, Milenkaya TM. [Diabetes Mellitus: retinopathy, nephropathy]. M.: Meditsina; 2001. 175 p. In Russian. 2.Jain A, Sarraf D, Fong D. Preventing diabetic retinopathy through control of systemic factors. Curr. Opin. Ophthalmol. 2003;14(6):389-94. 3.Veselovskaia ZF, Kindii TV. [Diagnostics of the preclinical stage and prediction of diabetic retinopathy progression]. Oftalmol Zh. 2001;1:13-6. In Ukrainean. 4.Pasechnikova NV, Naumenko VA, Zborovskaya AV. [Blood-retinal barrier state during diabetic retinopathy as per fluorometry data]. Oftalmol Zh. 2008; 5: 4-7. In Russian. 5.Poltorak VV, Blokh KO, Malashenko AM. [Experimental modeling of diabetes to study the specific effect of new antidiabetic agents]. Metodological recommendations. Kharkov; 1991. 19 p. In Russian. 6.Barber AJ. A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog. In Neuro-Psychopharm. & Biol. Psych. 2003;27:283 – 90. 7.Efimov A, Skorobonskaya N, Zuev N. [Diabetic neuropathy]. Liky Ukraine. 2005;(3):21–5. In Russian. 8.Leus NF. [Metabolic development mechanisms and pharmacotherapy aspects of diabetic retinopathy]. Oftalmol Zh. 2003; 5: 75-80. In Russian. 9.Forbes JM, Yee LTL, Thallas V. Advanced glycation end product interventions reduce diabetes-accelerated atherosclerosis. Diabetes. 2004;53:1813-23. 10.Kravchuk EA. [The role of free radical oxidation in pathogenesis of eye diseases]. Vestnik oftalmologii. 2004; 5: 48-51. In Russian. 11.Moroz OA. [Membrane-protective effect of quercetin and lipoate on the retina in streptozotocin diabetes]. Oftalmol Zh.2014;3:76-80. In Russian. 12.Bayness JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405-12. 13.Feillet-Coudry C, Rock E, Coudry C. Lipid peroxidation and antioxidant status in experimental diabetes. ClinChimActa. 1999;284:31 – 43. 14.Gutteridge JMC. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin. Chem. 1995;41:1819-28. 15.Kesavulu MM, Rao BK, Giri R. Lipid peroxidation and antioxidant enzyme status in Type 2 diabetic with coronary heart disease. Diabetes Res. Clin. Pract. 2001;53:33-9. 16.Reyk DM, Gillies MC, Davies MJ. The retina: oxidative stress and diabetes. Redox Rep. 2003;8 (4):187-92. 17.Stitt AW, Curtis TM. Advanced glycation and retinal pathology during diabetes. Farmac. Rep. 2006;57:156 – 68. 18.Pavlyuchenko KP, Oleinik TV. [The sdudy of lipid perioxidation products in the development of streptozotocine-induced diabetes]. Vestnik neotlozhnoi I vosstanovitelnoi meditsiny. 2005; 6(3): 510-3. In Russian. 19.Leus NF, Oleinik TV, Kolomiichuk SG. [Effect of vitamin B1 preparations (cocarboxylase and benfotiamine) on biophysical and metabolic processes in the retina and plasma albino rats with streptozotocin diabetes]. Oftalmol Zh. 2007;2:70–75. In Russian. 20.Mogilevskii SYu, Chuyko AL. [Influence of various vitamin В6 forms on the level of lipid perioxadation in the retina of the animal in streptozotocine diabetes]. Problemy ekologicheskoi I meditsinskoi genetiki I klinicheskoi immunologii. 2010;6(103):228-39. In Russian. 21.Andreeva LI, Kozhemiakin LA, Kishkun AA. [Modification of the method of determining lipid peroxidation in a test using thiobarbituric acid]. Lab Delo. 1988;(11):41-3. In Russian. 22.Skornyakov VI, Kozhemyakin LA, Smirnov VV et al. [Lipid perioxidation products in cerebro-spinal fluid in patients with cerebrocranial trauma]. Lab. Delo. 1988;8:14-6. In Russian. 23.Stal'naya ID Method of determination of conjugated diene of unsaturated higher fatty acid. Modern methods in biology, ed. VN Orekhovich. M.: Meditsina;1977. 63-4. In Russian. 24.Nasledov A. [SPSS computer data analysis in psychology and social sciences]. Spb.: Peter; 2005. 416 p. In Russian.
|