J.ophthalmol.(Ukraine).2016;4:23-28.
|
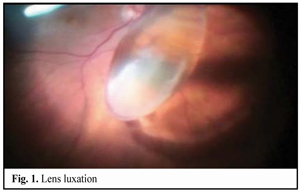
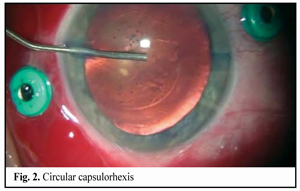
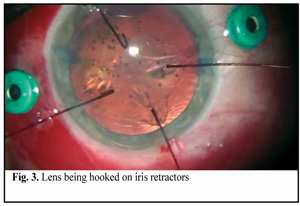
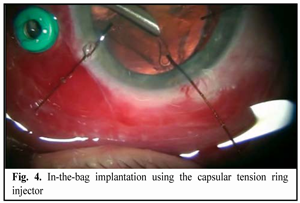
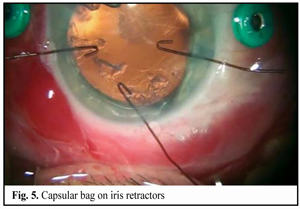
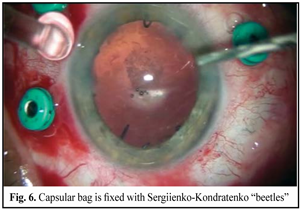
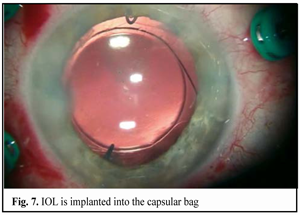
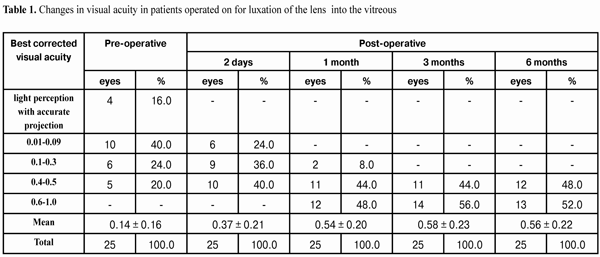
https://doi.org/10.31288/oftalmolzh201642328 Minimally invasive technique for the correction of luxation of the lens into the vitreous and in-the-bag IOL implantation D.V. Zhmuryk, Cand Sc (Med) Kyiv Municipal Clinical Eye Hospital “Eye Microsurgery Center” Kyiv, Ukraine E-mail: visus@ukr.net Background: The availability of a variety of the methods proposed for the removal of luxated lens and implantation of the intraocular lens (IOL) can make us conclude that, currently, there is no unified surgical tactics for this disorder. Purpose: To develop a minimally invasive technique for the correction of luxation of the lens into the vitreous and in-the-bag IOL implantation. Materials and Methods: Twenty five patients were operated on for the lens luxation secondary to ocular contusion and followed up for a mean of 3 years during September 2011 to February 2016 at the Kyiv Eye Microsurgery Center. Preoperatively, the mean best-corrected visual acuity (BCVA) was 0.14 ±0.16 (range, light perception with accurate projection to 0.5), and the mean intraocular pressure (IOP) was 19.74 ± 2.64 mmHg (range, 18 to 27 mmHg). Results: At day 2, month 1, month 3, month 6, and month 12 following surgery, the BCVA was 0.37 ± 0.21, 0.54 ± 0.20, 0.58 ± 0.23, 0.56 ± 0.22 and 0.57 ± 0.19, respectively (Table 1), and surgically induced astigmatism averaged 1.31 ± 0.62 D, 0.95 ± 0.36 D, 0.75 ± 0.31 D, 0.61 ± 0.25 D and 0.56 ± 0.22 D, respectively. Conclusions: The technique presented allows the surgeon to remove the lens through a small self-sealing incision, with a low incidence of intraoperative and postoperative complications; to implant any soft IOL designs; to restore the normal structure of the eye; and to make an IOL stable and well-centered, with surgically induced astigmatism averaged 0.56±0.22 D and preservation of or improvement in visual acuity to 0.5 or better at the 12 months. Key words: luxation of the lens into the vitreous, surgical treatment, in-the-bag IOL implantation Introduction The evolution of eye surgery over the recent decades has been characterized by the emphasis on the development of minimally invasive technologies. In particular, the trend to minimizing the surgical trauma can be traced in vitreoretinal surgery. In our opinion, the 25G has become the reference standard for vitreoretinal surgery. Although the 25-G approach can be used in the management of almost any vitreoretinal disorder, using it for the management of luxation and grade III [1] subluxation of the lens into the vitreous, as well as severe ocular trauma with intraocular foreign body poses some difficulties. Modern techniques for cataract surgery allow for excellent visual and anatomical outcomes. However, in difficult cases (luxation and subluxation of the lens into the vitreous), selection of the technique for removal of the lens, fixation of the capsular bag, as well as IOL implantation and centration remains a challenge. In grade I [1] subluxation, implantation of a standard (or classical) central tension ring (CTR) will be sufficient to fixate the capsular bag in place. In grades II-III [1] subluxation, implantation of a Malyugin CTR, Cionni ring, Sergiienko-Kondratenko capsular bag fixer (“beetle”), Ahmed segments [2, 3], or capsule anchor will be sufficient for this purpose. Opinions of eye surgeons are more mixed when it comes to surgical tactics in cases of complete luxation of the lens into the vitreous. With regard to the removal of the lens, it has been proposed (a) in the luxated lens nuclear density grade 1 to 3+, to perform posterior subtotal pars plana vitrectomy, free up the lens material from vitreous fibers, and to perform phacofragmentation or lensectomy in the vitreous cavity, whereas (b) in the luxated lens nuclear density grade 4+ or 5+, to perform intracapsular extraction of the lens by anterior approach after posterior vitrectomy [4]. Suturing the IOL in the iridociliary sulcus, transscleral fixation, suturing the IOL to the iris, and implantation of anterior chamber IOLs or iris-clip IOLs have been proposed with regard to IOL implantation. The availability of a variety of the methods proposed for the removal of luxated lens and implantation of the intraocular lens (IOL) can make us conclude that, currently, there is no unified surgical tactics for this disorder. The study purpose was to develop a minimally invasive technique for the correction of luxation of the lens into the vitreous followed by IOL implantation into the capsular bag. Materials and Methods Twenty five patients (25 eyes; 18 men (72%) and 7 women (28%); age, 46.2 ± 3 years, range, 18 to 63 years) diagnosed with luxation of the lens into the vitreous after ocular contusion were operated on and followed up during September 2011 to February 2016 at the Kyiv Municipal Clinical Eye Hospital “Eye Microsurgery Center”. At baseline (preoperative), in 12 eyes, 6 eyes, 4 eyes, and 3 eyes, the time since the contusion was < 1 month, 1-3 months, 4-6 months, and > 6 months, respectively. Five patients (20%) were diagnosed with pathologic myopia. Four of the patients (16%) had a total vitreous hemorrhage and 8 (32%) had a partital vitreous hemorrhage. Four of the patients (16%) had no fellow eye. Postoperative follow-up was 3 months to 4 years. Preoperatively, 4 eyes (16%) had BCVA of light perception with accurate projection, 10 eyes (40%) had BCVA of 0.01-0.09, and 5 eyes (20%) had BCVA of 0.4-0.5 (Table 1), with the mean BCVA of 0.14±0.16. Preoperatively and postoperatively, all patients underwent visual acuity assessment, perimetry, tonometry, tonography, biomicroscopy, direct and indirect binocular ophthalmoscopy. IOL calculations were performed before and after IOL implantation. Intraocular pressure (IOP) was 19.74 ± 2.64 mmH (range, 18 to 27 mmHg). Surgical technique Local subtenon anesthesia was used in all cases. Surgery was performed under control of operating microscope OMS-800 OFFISS (Topcon, Japan) using the Constellation Vision System (Alcon Laboratories, Inc., Fort Worth, TX). Diprospan (7 mg/ml; 2 mg of betamethasone dipropionate and 5 mg of betamethasone disodium phosphate) was injected into the vitreous cavity to afford better visualization of the vitreous base and better anti-inflammatory effect. After posterior 25G+ (27G+) vitrectomy, diprospan-contrasted posterior hyaloid was removed, and dislocated lens was freed up from vitreous fibers (Fig. 1). Then perfluorocarbon liquids were infused to float the nucleus anteriorly, until it was lodged at the vitreous base between the surface of the perfluorocarbon and the iris. Gripping forceps were used to create a circular capsulorhexis through the paracentesis (Fig. 2). The capsular bag was hold with four hook-type iris retractors (Fig. 3), or with three to four iridocapsular Malyugin retractors (“leaves”). After hydrodissection, a 13-mm diameter CTR was implanted in the capsular bag (Fig. 3). Phacoemulsification was performed (Fig. 5) through the 2.2-mm corneal tunnel using a phacochop technique. Either a Sergiienko-Kondratenko capsular bag fixer (“beetle”) or two Malyugin CTRs were used to stabilize the capsular bag. Three “beetles” were sutured at three meridians spaced 120? apart (Fig. 6). While the suture stabilization of the capsular bag was in process, retractors were removed one by one from the eye. A flexible IOL was implanted into the capsular bag using an injector (Fig. 7). Viscoelastic material and PFCL were thoroughly removed from the anterior chamber and the vitreous cavity, respectively. At the end of surgery, the ports were removed.
Results and Discussion At day 2, month 1, month 3, month 6, and month 12 following surgery, the BCVA was 0.37 ± 0.21, 0.54 ± 0.20, 0.58 ± 0.23, 0.56 ± 0.22 and 0.57 ± 0.19, respectively (Table 1), and surgically induced astigmatism averaged 1.31 ± 0.62 D, 0.95 ± 0.36 D, 0.75 ± 0.31 D, 0.61 ± 0.25 D and 0.56 ± 0.22 D, respectively. Visual fields were normal in all the cases.
The safety of the technique presented was assessed through occurrence of complications during surgery and follow-up. Early postoperative complications included mild iridocyclitis (2 cases (8%)), which required anti-inflammatory medications; ocular hypertension (42 cases (16%)), which was managed conservatively during 1-3 days; and descemetitis (2 cases (8%)). Cystoid macular edema was a late postoperative complication in 2 cases (8%), and resolved following conservative therapy. The lensectomy and phacofragmentation in the vitreous cavity has some particularities and difficulties. To remove the luxated lens with nuclear density grade 1 to 3+, initially, the capsular bag is removed, and, subsequently, depending on the particular nuclear density grade, either transciliary lensectomy or phacofragmentation is performed. If lensectomy proves to be inefficacious, phacofragmentation is performed. However, phacofragmentation requires a 20-G incision, and, during phacofragmentation, ultrasound energy is dissipated into the vitreous cavity, resulting in retinal damage. In 1997, Livshitz introduced the notion of “equivalent time of ultrasound exposure” for the assessment of the influence of ultrasound on the posterior eye, and recommended 8.8 W and three minutes as the power and the equivalent exposure time limits not to be exceeded in the clinical setting [5]. Berger and colleagues [6] observed 7-°C and 35-°C increases in the temperature of the tissue surrounding the lens as a result of two-minute delivery of 100% power of phaco, with and without the use of irrigation system, respectively. Bessarabova and Komarova [7] investigated the effect of phaco ultrasound on the detached and attached retina, and proved that phacoemulsification can be used in vitreoretinal surgery with certain limitations. A disadvantage of lensvitrectomy for the removal of luxated lens with a hard nucleus is the difficulty in stabilizing and holding the lens with a vitreous cutter during its removal from the vitreous cavity. Perfluorocarbon liquids have been used to facilitate surgery in patients with these conditions over the past three decades [8, 9]. Perfluorocarbon liquids have high density, low surface tension, low viscosity, and high gas-dissolving capacity; they are twice as heavy as water, water-insoluble and have a low dissolving power for most of organic solvents. Their high specific gravity is an advantage for use in ocular surgery. In the luxated lens nuclear density grade 4 to 5+, in a traditional approach to surgery for lens luxation, it is advised to extract the lens through the sclerocorneal incision (an anterior approach). This approach is justified in the absence of retinal detachment, since it allows avoiding damage from ultrasound and potential retinal tractions. However, the surgeon’s task becomes much more complicated if the patient with luxation of the lens into the vitreous also has a diagnosis of retinal detachment, especially in the luxated lens nuclear density grade 4 to 5+. The main disadvantage of the intracapsular extraction of the dislocated lens is the requirement for a large incision (up to 15 mm), that have a rather high potential for trauma [8]. Such a “total surgery” with a significant depressurization of the eye results in a significant reduction in the success rate of surgery for retinal detachment. In different techniques for making a large sclerocorneal incision, corneal astigmatism values can be as high as 3.0 D even a year after surgery and as high as 4.0-6.0 D at day 3 after surgery, making ophthalmoscopy difficult to perform [4]. Making a large sclerocorneal incision results in a loss of spherical shape of the globe and edema of wound margins; this prevents sufficient intraoperative visualization of the retina and vitreous, and makes binocular ophthalmoscopy difficult to perform. Even if a large incision is sealed with sutures, its presence creates a potential for gas and liquid leakage after increases in intraocular pressure (IOP) during retinal detachment surgery. Temporary increases in introperative IOP may result in sutures ripping out, anterior chamber shallowing, and iris suture entrapment. Given a high expansion factor of fluorine-containing gases, and secondary ocular hypertension following silicon injection, similar complications may be observed during the postoperative period. Poor adaptation to intra- and post-operative increases in the IOP and difficulties in performance of adequate intraoperative ophthalmoscopy challenges the appropriateness of making a large cataract incision during combined surgery for luxated dense cataract with retinal detachment. Another substantial disadvantage of the large incision approach to combined surgery is the impossibility of performance of the intraocular correction of aphakia at this stage. Opinions are more mixed when it comes to the intraocular correction, and there is no universal IOL or universal IOL implantation approach to lens luxation surgery. It is customary to extract the luxated lens along with the capsule. While using this customary approach, the surgeon wins the advantages of putative ease of surgery and low surgery time, but faces with the problem of choice with regard to the method of the intraocular correction of surgical aphakia. The anterior chamber (AC) and pupil-fixated IOLs used earlier were abandoned due to technical difficulties of implanting them and numerous complications, and are of historical interest only. Contact between the fixation elements of AC IOLs and the AC angle structures often results in the development of corneal dystrophy or secondary glaucoma. The use of pupil-fixated IOLs was limited by compromised iris diaphragm and risk of the dislocation of either the fixation elements or the entire IOL. The Sergiienko’s iridocapsular-fixated “gymnast” IOL design, commonly used earlier in this country, is rather seldom used today as it requires a large sclerocorneal incision and causes rigidity of the pupil, compromised pupil diaphragm function and uveal reactions. Currently, a scleral or iris-fixated three-piece posterior chamber IOL is the most commonly used design for intraocular correction in the absence of capsular support. However, IOL suturing in the iridociliary sulcus is technically difficult, laborious, has a high potential for trauma, is accompanied by hemorrhages from the ciliary body, and requires additional conjunctival and scleral incisions. Suture fixed IOL is prone to decentration and dislocation with regard to the frontal plane [2, 10]. The intrascleral haptic-fixated IOL provides a higher degree of stability [4, 11], but the implantation is more traumatic to the eye, compared to the iris-fixated IOL. Morphological studies have shown that in posterior chamber IOLs with ciliary sulcus fixation, the most marked changes are observed in the iris and ciliary processes, which is manifested in the desquamation and focal proliferation of the pigment epithelium of the iris and ciliary processes, with the development of cicatricial masses [11]. Iris-sutured IOL fixation is simpler and less traumatic compared to ciliary sulcus fixation [3, 12]. In iris-sutured fixation, lens centration is problematic, since haptics are sutured to the iris in a blind fashion. Due to the haptic design, there is only point contact between the haptic and the iris, with a high potential for instability with respect to the frontal plane. In addition, displacement of the haptics is possible. Tightening the slip knot in an attempt to reposition the IOL may result in sutures tearing through tissue [13]. The bag-sparing technique we propose here for the extraction of lens luxated into the vitreous allows us to make such surgery more comparable to phacoemulsification in terms of small invasiveness, predictability and a protocol approach. We could not find a description of a similar technique for capsular bag suturing with implantation of IOL in luxation of the lens into the vitreous. Although the technique is difficult, it has the following benefits. First, it is minimally invasive and traumatic, which results in a low amount of induced astigmatism and short rehabilitation period. Second, phacoemulsification takes place not in the vitreous cavity (with subsequent migration of nuclear fragments and risk of retinal damage and detachment), but in the posterior chamber and capsular bag, with all the benefits of standard phacoemulsification. Third, the technique offers good IOL centration and stable IOL position. And last, it offers the potential to implant any posterior chamber design (including multifocal and toric) of IOL into the capsular bag. Analysis of the outcomes of surgical treatment for luxation of the lens into the vitreous showed that intraoperational complications were present only when we were on a “discovery curve” for a procedure. Fixation of the nucleus in the pupil opening with subsequent creation of circular capsulorhexis is the most difficult phase of the technique. A posterior capsule tear during phacoemulsification was the most common problem. To address the problem, we removed the lens residue, and either sutured a three-piece posterior chamber IOL in the ciliary sulcus (or to the iris), or performed intrascleral IOL fixation. Dropped nuclear fragments were a rare complication. Ciliary body hemorrhage occurred during suturing of the “beetles” or Malyugin CTRs, and was managed by increasing the IOP. Conclusions First, the bag-sparing technique presented here for the extraction of the lens luxated into the vitreous is efficacious, allowing the surgeon (1) to remove the lens through a small self-sealing incision, with a low incidence of intraoperative and postoperative complications, (2) to implant any soft IOL designs, and (3) to restore the normal structure of the eye with preservation of the capsular bag. Second, in patients with the lens luxated into the vitreous, the technique allows making an IOL stable and well-centered, with surgically induced astigmatism averaged 0.56±0.22 D and preservation of or improvement in visual acuity to 0.5 or better at the 12 months.
References 1.Pashtaev NP. [Lens dislocation: Classification and modern management strategies]. 2.In: [Fedorov SN, editor. Current issues of surgery of the lens, vitreous and retina: A Collection of Works]. Moscow: Eye Microsurgery Research Institute; 1986. p. 34–7. Russian 3.Miroshnikov VV. [Technique for correction of aphakia in the absence of capsular support]. Modern Technologies in Ophthalmic Surgery. 2015;4(8):72–3 Russian 4.Pershin KB. [Pseudophakia surgery]. In: [Proceedings of the 5th Russian National Round Table on Macula]; 2012 May 18-20; Rostov-on-Don (Russia); 2012. p.220–6 Russian 5.Gabor SG, Pavlidis MM. Sutureless intrascleral posterior chamber intraocular lens fixation. J Cataract Refract Surg. 2007 Nov;33(11):1851–4 6.Livshitz SA. [Developing optimum ultrasound exposure parameters for phacoemulsification with IOL implantation]. [Abstract of Cand Sc (Med) thesis]. Moscow: Eye Microsurgery Complex; 1997. 20 p. Russian 7.Berger RE. Intraocular lens placement after removal of a subluxated lens. Ophthalmic Surg. 1994 Sep-Oct;25(9):657–8 8.Bessarabov AN, Komatova MG. [Mathematical model for the effect of phaco ultrasound the detached and attached retina]. In: [Proceeding of the 1st Eurasian Conference on Ophthalmic Surgery]. Ekaterinburg, 1998. p.8–9. Russian 9.Chang S, Zimmerman NJ, Iwamoto T et al. Experimental vitreousreplacement with perfluorotributylamine. Am J Ophthalmol. 1987 Jan 15;103(1):29–37 10.Miyamoto K, Refojo MF, Tolentino FI, et al. Perfluoroether liquid as a long-term vitreous substitute: an experimental study. Retina. 1984; 4:264–8 11.Monteiro M, Marinho A, Borges S, et al. Scleral fixation in eyes with loss of capsule or zonule support. J Cataract Refract Surg. 2007 Apr;33(4):573–6 12.Kumar DA, Agarwal A, Prakash G, et al. Glued posterior chamber IOL in eyes with deficient capsular support: a retrospective analysis of 1-year post-operative outcomes. Eye (Lond). 2010 Jul;24(7):1143–8 13.Chang S, Lincoff H, Zimmerman NJ et al. Giant retinal tears. Surgical techniques and results using perfluorocarbon liquids. Arch Ophthalmol. 1989 May;107(5):761–6 14.Kaiura TL, Seedor JA, Koplin RS, et al. Complications arising from iris-fixated posterior chamber intraocular lenses. J Cataract Refract Surg. 2005 Dec;31(12):2420–2
|