J.ophthalmol.(Ukraine).2016;3:10-14.
|
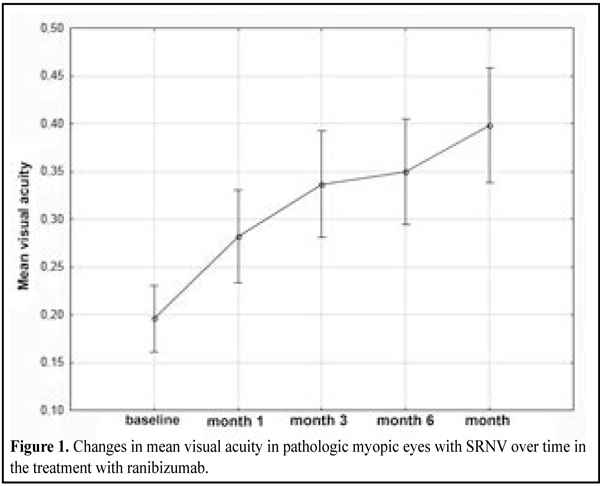
https://doi.org/10.31288/oftalmolzh201631014 Use of ranibizumab for the treatment of subretinal neovascularization in pathologic myopia: a prospective study О.M. Blavatska1 T.B. Kustryn2 A.R. Korol2, Dr Sc (Med) 1Danylo Halytsky Lviv National Medical University 2Filatov Institute of Eye Disease and Tissue Therapy, Odessa E-mail: laserfilatova@gmail.com Purpose: To determine the efficacy of ranibizumab as a treatment for subretinal neovascularization (SRNV) in pathologic myopia. Materials and Methods: This was an uncontrolled, prospective cohort study, involving 66 pathologic myopic eyes (65 patients) with SRNV. Patients were treated with 0.05-mL (0.5 mg) intravitreal ranibizumab and followed up over 12 months. The best-corrected visual acuity (BCVA) at 12 months was used as a primary end point. Safety, OCT retinal thickness at the fovea, activity of SRNV as assessed by fluorescein angiography, and the number of ranibizumab injections were secondary end points. Results: Compared with baseline, the visual acuity improved significantly at all follow-up points (р = 0.001). The mean BCVA was 0.2 (SD 0.13) at baseline and improved to 0.4 (SD 0.21) at month 12. The mean CRT was 313 µm (SD 82) at baseline and decreased to 244 µm (SD 31) at the end point (р = 0.0001). Conclusions: Intravitreal ranibizumab was found to be a safe and efficacious treatment for SRNV in pathologic myopia at the follow-up of 12 months. In routine clinical practice, the use of ranibizumab for the treatment of SRNV in pathologic myopia results in a statistically significant improvement in visual acuity and a regression in retinal edema. Key words:subretinal neovascularization, pathologic myopia, ocular disease, ranibizumab, angiogenesis inhibitors Introduction Subretinal neovascularization (SRNV) is one of the most common sequelae of high complicated myopia (HCM). The prevalence of pathologic myopia has been reported to be 0.9% to 3.1% among adult population. SRNV secondary to myopia, if untreated, has a poor visual acuity prognosis, with a reduction in visual acuity to 20/200 or worse in 95% of eyes [1, 2]. The risk of the development of myopic SRNV has been estimated to be as high as 5% to 11%. It has been reported that, with the presence of SRNV in the first eye, its development in the fellow eye occurs in 35% of cases within 8 years [3, 4]. Laser coagulation, transpupillary thermotherapy and photodynamic thermotherapy have been proposed as treatments for SRNV. It is noteworthy that although laser treatments have some efficacy in SRNV, the long-term results of these treatments are often unsatisfactory [1, 2, 5, 6]. The study purpose was to determine the efficacy of ranibizumab as a treatment for SRNV in pathologic myopic eyes. Materials and Methods This was an uncontrolled, prospective cohort study, involving 66 pathologic myopic eyes (65 patients) with subretinal neovascularization. Patients were treated with 0.05-mL (0.5 mg) intravitreal ranibizumab and followed up over 12 months. Inclusion criteria for this study were presence or history of pathologic myopia (defined as a spherical equivalent equal or higher than –6 diopters), recent (within 2 months) history of SRNV associated with pathologic myopia, and age of 18 years or more. We used a pro re nata (PRN) dosing strategy with two loading monthly doses and further injections, depending on the worsening of anatomic and functional measures (i.e., as required). Patients underwent visual acuity (VA) assessment, microscopic examination, macular optical coherence tomography (OCT) and fluorescein fundus angiography (FFA). FFA was performed both at baseline and on completion of the 12-month treatment course. No hypofluorescence at 12 months was considered as closure of neovascularization. Presence of hypofluorescence on FFA was considered as evidence of active SRNV. The best-corrected visual acuity (BCVA) at 12 months was used as a primary end point. OCT central retinal thickness (CRT), activity of SRNV as assessed by fluorescein angiography, the number of ranibizumab injections and safety were secondary end points. All patients were observed and treated at Ocular Laser Microsurgery Department of the Filatov Institute. Primary data base was developed, the data were statistically processed and diagrams were developed using Statistica 10.0 (StatSoft, Tulsa, OK). The T test was used to compare intragroup changes in VA, CRT and the number of injections over time, with the mean (M) and standard deviation (SD) being estimated. A P-value of < 0.05 was considered as statistically significant in all analyses. Results The mean age was 47.8 years (SD 14.2) and 55 patients (85%) were women. The predominant pattern was a classic SRNV in the macula. The SRNVs were juxtafoveal and subfoveal in 12 eyes (18%) and 54 eyes (82%), respectively. The mean BCVA was 0.2 (SD 0.13) at baseline and improved significantly to 0.3 (SD 0.19), (р=0.0001) at month 1 after the first ranibizumab injection. Compared to baseline, at month 2, the mean BCVA increased significantly to 0.32 (SD 0.14) (р=0.0001). At month 3 and month 6, the mean BCVA was 0.36 (SD 0.24) (р=0.0001) and 0.37 (SD 0.2) (р=0.001), respectively. Compared to baseline, at month 9 and month 12, the mean BCVA improved to 0.38 (SD 0.2) (р=0.001) and 0.4 (SD 0.21) (р=0.0001), respectively (Fig. 1).
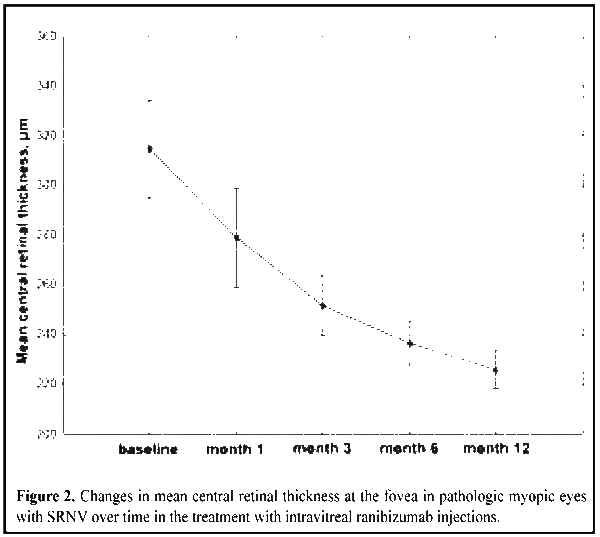
The mean CRT was 313 µm (SD 82) at baseline. It decreased statistically insignificantly by 28 µm at month 1 (р=0.1), and continued to decrease gradually until completion of the observation period. At the end point, the mean CRT decreased by 69 µm compared to baseline (Fig. 2).
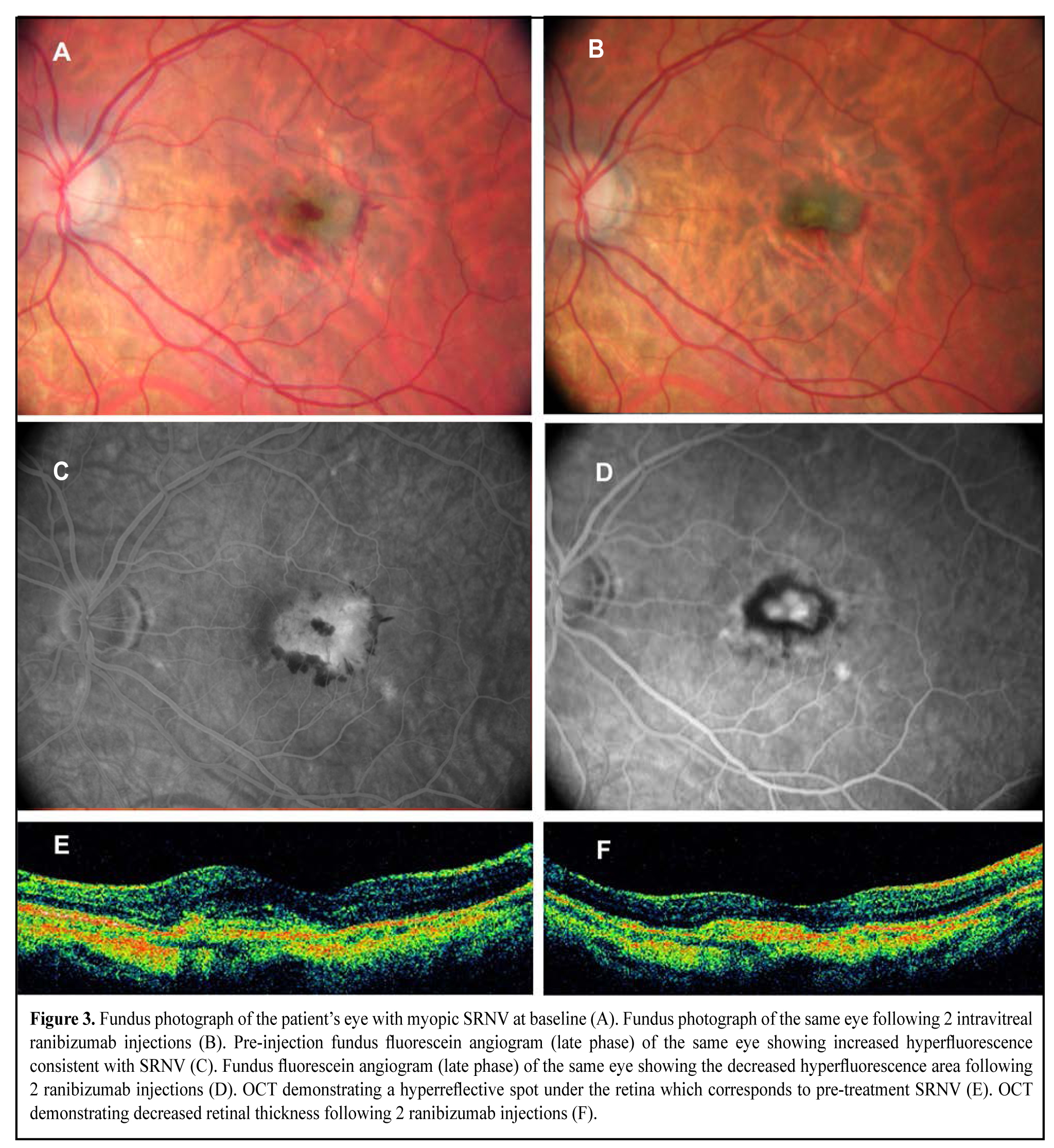
At month 12, the mean number of injections was 2.3 (SD 0.9), and the maximum number of injections per eye was 6.0. Although a recurrent SRNV developed in 2 eyes (2 patients), it was seen to resolve after additional ranibizumab injections (Fig. 3).
No case of endophthalmitis, uveitis, retinal detachment or cardiovascular sequelae was observed. Discussion Some disadvantages of treatments for SRNV associated with pathological myopia have been reported. Thus, although laser coagulation of subfoveal SRNV does inhibit the membrane activity, further damage to the superior neurosensory retina results in decreased visual acuity. It has been shown [7, 8] that the difference between the mean decrease in VA of myopic SRNV eyes treated with laser photocoagulation and non-treated eyes is statistically significant at two years and insignificant at five years. Jalkh and colleagues [9] have demonstrated that visual acuity improves in only 11%, stabilizes in 21%, and deteriorates in 68% of eyes at 29.2 months following laser photocoagulation for SRNV in degenerative myopia. Although transpupillary thermotherapy for myopic subretinal neovascularization contributes to closure of new vessels, it does not result in a long-term improvement in visual acuity [10]. In 2001, photodynamic therapy (PDT) with verteporfin was introduced as a safe, well-tolerated technique for the treatment of SRNV in pathologic myopia. More recently, it has been shown [11, 12] that 86% of the verteporfin-treated patients compared with 67% of the placebo-treated patients loose fewer than 15 letters, although the difference is statistically insignificant. Introduction of vascular endothelial growth factor (VEGF) inhibitors enabled ophthalmologists to save and improve visual acuity in patients with myopic SRNV. Numerous reports have demonstrated safety and efficacy of bevacizumab for myopic SRNV. Arias and colleagues [13] reported on a study of patients with SRNV secondary to PM in whom, at the 6-month follow-up after treatment with an average of 1.2 intravitreal bevacizumab injections, the mean visual acuity improved by 8.4 letters. Forty-one per cent of patients increased at least one line, and 17% increased more than six lines. Laud and co-authors [14] found that at the 7.3 month after treatment with intravitreal bevacizumab injections, the mean visual acuity in eyes with myopic SRNV improved by 1.5 lines. Baba and colleagues [5] have demonstrated that, at 24 months following treatment of myopic SRNV with either intravitreal bevacizumab or PDT, the former is more effective than the latter. Ikuno and co-authors [15] have observed that, at 12 months following treatment of 63 eyes with one to six injections (mean, 2.4 injections) for subretinal neovascularization attributable to pathological myopia, visual acuity improves in most of these eyes. The BCVA improved more than three lines in 25 eyes (40%), worsened more than three lines in three eyes (5%), and was unchanged in 35 (56%) eyes. Fluorescein leakage from the SRNV ceased in 30 eyes (48%), diminished in 28 (44%), and was unchanged in five (8%) eyes. The efficacy and safety of ranibizumab for myopic SRNV have been proven in RADIANCE, a recent international multicentre study [16] involving 116 patients, with a mean BCVA change of 13.8 ETDRS letters following three intravitreal ranibizumab injections at the follow-up of 12 months. Since we used the Shevaliov visual acuity chart, the difference in visual acuity charts makes it impossible to directly compare the findings of our study with this recent study. However, in our study, we observed an improvement in the mean BCVA from 0.2 (SD 0.13) to 0.4 (SD 0.21) following two intravitreal ranibizumab injections at the follow-up of 12 months, with the improvement being statistically significant. The changes in mean retinal thickness at the fovea over time were comparable in these two studies, with a reduction in CRT of 71.3 µm at month 12 in the RADIANCE study [16] compared to 69 µm at month 12 in our study, both reductions being statistically significant. Conclusions Intravitreal ranibizumab was found to be a safe and efficacious treatment for SRNV in high myopia at the follow-up of 12 months. Treatment with PRN intravitreal ranibizumab (mean, two injections) results in a statistically significant improvement in visual acuity and a regression in retinal edema in the macula. References
|