J.ophthalmol.(Ukraine).2016;3:49-54.
|
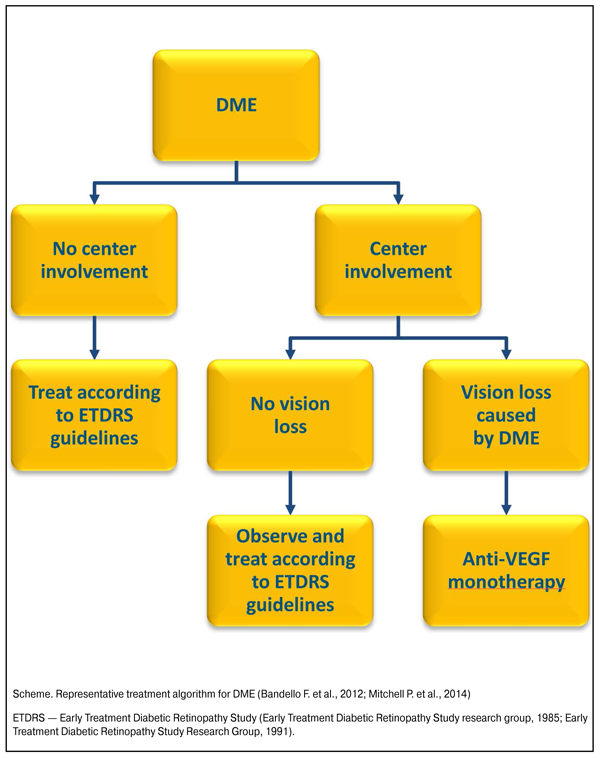
https://doi.org/10.31288/oftalmolzh201634954 Expert Consensus Optimizing management of patients with visual impairments as a result of diabetic macular edema. Role and place of the anti-VEGF-preparation aflibercept Moderator: Katz Todd, MD, Ophthalmologist, Retinal Specialist (New York, USA) Members: Vitovska Oksana P. — Professor, Head of the Ophthalmology Department of the National Medical University, Main ophthalmologist of the Ministry of Health of Ukraine (Kiev, Ukraine) Korol Andrii R. — Doctor of Medical Sciences, The Filatov Institute of Eye Diseases and Tissue Therapy of the National Academy of Medical Sciences (NAMS) of Ukraine, Head of laser department (Odessa, Ukraine) Mankovsky Borys M. — Professor, Corresponding Member of NAMS of Ukraine, Head of the Department of Diabetology of the Shupyk National Medical Academy of Postgraduate Education, Main endocrinologist of the Ministry of Health of Ukraine (Kiev, Ukraine) Naumenko Volodymyr A. — Professor, Deputy Director of The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine (Odessa, Ukraine) Pasyechnikova Nataliya V. — Professor, Corresponding member of NAMS of Ukraine, Director of The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine (Odessa, Ukraine) Ciardella Antonio P.— Associate Professor of Ophthalmology, University of Colorado Health Sciences Center, Denver (USA); Director of Ophthalmology, Azienda Ospedaliera-Universitaria, Policlinico (Bologna, Italy) International interdisciplinary symposium of ophthalmologists and endocrinologists and international interdisciplinary advisory board “Optimizing management of patients with visual impairments as a result of diabetic macular edema” took place in Kiev on November 7, 2015. According to the International Diabetes Federation — IDF (2015), some 415 million people worldwide, or 8.8% of adults aged 20-79, are estimated to have diabetes mellitus (DM). About 75% live in low- and middle-income countries. If these trends continue, by 2040, some 642 million people, or one adult in 10, will have diabetes. The largest increases will take place in the regions where developing economies are predominant. IDF also estimates that as many as 193 million people worldwide, or close to half of all people with diabetes (46,5%), are unaware of their disease. Most of these cases are type 2 diabetes. The earlier a person is diagnosed and management of diabetes begins, the better the chances of preventing harmful and costly complications (International Diabetes Federation — IDF, 2015). Diabetic retinopathy (DR) is one of the main microvascular complications of diabetes. The disease is progressive, developing from an initial ‘non-proliferative’ state to pre-proliferative and proliferative disease. Diabetic macular edema (DME) can occur in individuals with any stage of diabetic retinopathy. DR and DME are leading causes of blindness in the working-age population of most developed countries (Ciulla T.A. et al., 2003). Data of the prevalence of DME can vary widely. According to some estimations DME may affect ~7% of people with diabetes, and ~23% patients with DR (Ding J., Wong T.Y., 2012; Yau J.W. et al., 2012). As the global prevalence of diabetes is expected to rise significantly, the burden of DME will increase proportionately. Vision loss from DR/DME can have a significant impact on quality of life and the ability to perform daily activities and, importantly, may also compromise the ability of the patient to manage their underlying diabetes. According to some studies, visual loss is the main worry in diabetic patients (Coyne K.S. et al., 2004; Luckie R. et al.; DARTS/MEMO Collaboration, 2007). In patients with diabetes, the primary mechanism responsible for vision loss is centrally involved DME or clinically significant macular edema. The risk of developing visual loss increases as retinopathy becomes more severe, also because the incidence of DME increases. Approximately one third of persons with clinically significant macular edema who are untreated have a significant loss of central vision within 3 years (Davidson J.A. et al., 2007). Many healthcare systems in developed countries have established approaches to ensure regular screening for DR, typically with annual retinal examinations and/or fundus photography. The results from such screening may prompt specialist management with varying degrees of urgency. Control of glycaemia, dyslipidemia and hypertension is essential for the management of diabetes, and is associated with the prevention and delay of onset of diabetes-related macrovascular and microvascular complications. Although well-managed diabetes can reduce the risk of developing or slow the progression of macro- and microvascular complications, even patients with good control of their disease often develop ophthalmic complications such as DR and DME. Laser photocoagulation (focal, grid or panretinal) have been widely recommended as the standard of care for DR/DME since the 1980s. At present, for mild-to-moderate non-proliferative DR in the absence of any clinically significant DME, ongoing monitoring and no treatment is usually recommended. In proliferative DR, active laser treatment is indicated to prevent further progression of the disease. Pan-retinal laser photocoagulation may be recommended as soon as proliferative disease is detected, to prevent vision loss and the need for vitrectomy (American Academy of Ophthalmology Retina/Vitreous Panel, 2014; International Council of Ophthalmology, 2014). In DME laser photocoagulation may be effective at stabilizing vision, but does not result in a gain in vision. In addition, laser is not without side effects. Foveal burns, visual field defects, retinal fibrosis and laser scars have been reported (Ford J.A. et al., 2013). Intravitreal corticosteroid use considered as alternative to laser photocoagulation; however, mixed results in randomized, controlled trials and significant increases in adverse events such as cataracts and increased intraocular pressure limit the use of this approach (Grover D. et al., 2008; Ford J.A. et al., 2013). The unmet need of a treatment that can not only stabilize but also improve vision in patients with retinal vascular pathology, including DME, has led to the development and introduction in clinical practice from 2000s of intravitreal anti-VEGF (Vascular Endothelial Growth Factor) therapies. In the Diabetic Retinopathy Guidelines of The Royal College of Ophthalmologists (2012) intravitreal anti-VEGF injections are considered the new gold standard of therapy for eyes with centre-involving macular oedema and reduced vision (The Royal College of Ophthalmologists, 2012). According to the actual evidence-based clinical practice guidelines on the management of patients with DR/DME (Bandello F. et al., 2012; The Royal College of Ophthalmologists, 2012; American Academy of Ophthalmology Retina/Vitreous Panel, 2014; International Council of Ophthalmology, 2014), it is now appropriate to subdivide DME according to involvement at the center of the macula, because the risk of visual loss and the need for treatment is greater when the center is involved; and for DME with centre involvement and associated vision loss due to DME, monotherapy with intravitreal anti-VEGF agents considered as the initial treatment choice (Scheme).
According to the American Society of Retina Specialists Preference and Trends (PAT) Survey (2013), 90% of retinal specialists in the United States reported using anti-VEGF therapy for initial management of vision loss from DME involving the macular center (Diabetic Retinopathy Clinical Research Network, Wells J.A. et al., 2015). According to the Anatomical Therapeutic Chemical classification system (ATC), recommended by World Health Organization, anti-VEGF agents for intravitreal injections attributed to the group S01L A — Antineovascularisation agents (S01L - Ocular vascular disorder agents; S01- Ophthalmologicals; S - Sensory organs). Aflibercept solution for intravitreal injections (EYLEA) — modern anti-VEGF-preparation, have been applying in the world ophthalmological practice since 2011. Aflibercept is a recombinant fusion protein consisting of portions of human VEGF receptor 1 and 2 extracellular domains fused to the Fc portion of human IgG1. Unlike its predecessor, aflibercept acts as a soluble decoy receptor (VEGF Trap) that binds VEGF-A, VEGF-B and Placental Growth Factor (PlGF) with higher affinity than their natural receptors, and thereby can inhibit the binding and activation of these cognate VEGF receptors (Papadopoulos N. et al., 2012). The mean duration of VEGF suppression in aqueous humor specimens of patients with neovascular age-related macular degeneration after intravitreal aflibercept injections was 71±18 days (Fauser S. et al., 2014), whereas after intravitreal injections of its predecessor — the anti-VEGF-preparation ranibizumab — 36,4±6,7 days (Muether P.S. et al., 2013). Scientific program for aflibercept investigation in DME include clinical trials DA VINCI (phase II), VIVID and VISTA (phase III), VIVIDEast (phase III) and VIVIDJapan (open non-comparative study). In the key large-scale phase III randomized clinical trials VIVID (Europe — Austria, Czech Republic, Denmark, France, Germany, Hungary, Italy, Poland, and Spain, as well as Australia and Japan) and VISTA (USA) intravitreal aflibercept injections given every 8 weeks after 5 initial monthly doses demonstrated high efficacy in functional (best-corrected visual acuity/BCVA) and anatomic (central subfield thickness (CST) as determined by optical coherence tomography/OCT) endpoints at week 52 (Korobelnik J.F. et al., 2014). Mean gain of >1 line (5 letters) of vision observed after 1st dose, mean gain of 2 lines — after 6 injections, and 1/3 of patients gained ?3 lines (?15 letters) at 52 weeks. Also, near 1/3 of eyes treated with aflibercept had a 2-step improvement in Diabetic Retinopathy Severity Scale (DRSS) at week 52. In both VISTA and VIVID, the 52-week visual and anatomic effects of intravitreal aflibercept injections were sustained through week 100 (Brown D.M. et al., 2015). Clinical trials of aflibercept in DME demonstrated possibility to effectively apply it every 8 weeks after 5 initial monthly injections (Korobelnik J.F. et al., 2014; Brown D.M. et al., 2015). In the independent and first direct comparison study «Protocol T», performed by The Diabetic Retinopathy Clinical Research Network (DRCR.net) in the USA, intravitreous aflibercept was the most effective in comparison with other anti-VEGF agents in the visual and anatomic (central retinal thickness) indices improvement in the subgroup of DME patients with worse levels of initial visual acuity (<20/40) (Diabetic Retinopathy Clinical Research Network, Wells J.A. et al., 2015). At present, aflibercept solution for intravitreal injections (EYLEA) registered in Ukraine for adult patients with: -neovascular (wet) age-related macular degeneration (since 09.11.2012); -visual impairment due to macular edema secondary to retinal vein occlusion (RVO) —central RVO (since 23.09.2013) or branch RVO (since 14.12.2015); -visual impairment due to diabetic macular edema (since 02.04.2015). EYLEA included in the Ukrainian State Formulary of Medicines (8th edition, 2016). On the Advisory Board experts have reached interdisciplinary consensus on how to optimize the management of patients with visual impairments as a result of DME, as well as the role and the place of anti-VEGF-therapy with aflibercept in such category of patients. 1.Diabetes is important and growing problem in Ukraine. 2.DR and DME — important manifestations that can lead to vision loss. 3.Global diabetic population is growing with commensurate growth of the DR/DME population. 4.DR is one of the main reasons of substantial vision loss and disability in patients of working age in Ukraine. 5.A steady increase in the DR prevalence and incidence is registering during the last years in Ukraine. 6.The DR prevalence and incidence indices in Ukraine require in-depth study. 7.Efficacy of the DR treatment in the first place depends on its timely detection. 8.Patient should be examined by ophthalmologist immediately (or as soon as possible) after the diagnostics of DM. 9.Screening for DR according to the Ukrainian national guidelines on the management of patients with DM is absolutely mandatory in all patients with DM and should be actively encouraged and promoted (Table). 10.In case of sudden vision loss or any ocular complain in patients with diabetes ophthalmological examination should be performed immediately, regardless of the term of next visit to ophthalmologist. 11.Focal/grid macular laser has historically dominated DME management offering an advantage over natural history in ability to maintain vision. 12.In patients with symptomatic visual loss, treatment for DME in the world is shifting principally toward intravitreal anti-VEGF therapy. 13.Studies of anti-VEGF therapies in DME patients have shown high efficacy with respect to the visual outcomes. 14.The phase III VIVID and VISTA trials demonstrated high efficacy of aflibercept in patients with DME, with substantial improvements observed in both visual and anatomical endpoints. 15.In the independent direct comparison DRCR.net study “Protocol T” aflibercept was found to be one of the most effective anti-VEGF-preparation. 16.Late treatment of DME may lead to the irreversible loss of central vision. 17.3 different therapeutic strategies were formed abroad depending on the severity of DME and amount of vision loss: -per label: 5 loading doses (monthly injections) followed by bi-monthly injections in the first year, as needed (pro re nata — PRN) in the second year; -3 loading doses followed by PRN; -PRN from the start (“Protocol T”). 18.According to the Ukrainian experience, anti-VEGF agents are effective and safe for DME treatment. 19.PRN scheme without loading dose is substantiated for anti-VEGF therapy in Ukraine. 20.It is necessary to consider fluorescent angiography and OCT examination in patients with DME before the treatment initiation. 21.In Ukraine the existing protocols of diagnostics, treatment and monitoring of patients with DR/DME require an updating with new data on the diagnostic significance of OCT and treatment efficacy of intravitreal anti-VEGF-preparations. 22.It is necessary to initiate the formation of the interdisciplinary task force with the aim to elaborate modern unified clinical protocols “Diabetic retinopathy” with inclusion of the new evidence-based approaches to the diagnostics and treatment in this field. 23.The joint educational activities of endocrinologists and ophthalmologists is strongly encouraged. 24.Collaboration of diabetologists and ophthalmologists is the cornerstone of the successful diagnostics and treatment of DR/DME and prevention of blindness. References 1.American Academy of Ophthalmology Retina/Vitreous Panel (2014) Preferred Practice Pattern® Guidelines. Diabetic Retinopathy. San Francisco, CA: American Academy of Ophthalmology. Available at: http://www.aao.org/preferred-practice-pattern/diabetic-retinopathy-ppp--.... Accessed February 7, 2015. 2.Bandello F., Cunha-Vaz J., Chong N.V. et al. (2012) New approaches for the treatment of diabetic macular oedema: recommendations by an expert panel. Eye (Lond.), 26(4): 485–493. 3.Brown D.M., Schmidt-Erfurth U., Do D.V. et al. (2015) Intravitreal Aflibercept for Diabetic Macular Edema: 100-Week Results From the VISTA and VIVID Studies. Ophthalmology, 122(10): 2044–2052. 4.Ciulla T.A., Amador A.G., Zinman B. (2003) Diabetic retinopathy and diabetic macular edema: pathophysiology, screening, and novel therapies. Diabetes Care, 26(9): 2653–2664. 5.Coyne K.S., Margolis M.K., Kennedy-Martin T. et al. (2004) The impact of diabetic retinopathy: perspectives from patient focus groups. Fam. Pract., 21(4): 447–453. 6.Davidson J.A., Ciulla T.A., McGill J.B. et al. (2007) How the diabetic eye loses vision. Endocrine, 32(1): 107–116. 7.Diabetic Retinopathy Clinical Research Network, Wells J.A., Glassman A.R., Ayala A.R. et al. (2015) Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N. Engl. J. Med., 372(13): 1193–1203). 8.Ding J., Wong T.Y. (2012) Current epidemiology of diabetic retinopathy and diabetic macular edema. Curr. Diab. Rep., 12(4): 346–354. 9.Early Treatment Diabetic Retinopathy Study research group (1985) Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch. Ophthalmol., 103(12): 1796–1806. 10.Early Treatment Diabetic Retinopathy Study Research Group (1991) Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Ophthalmology, 98(5 Suppl.): 766–785. 11.Fauser S., Schwabecker V., Muether P.S. (2014) Suppression of intraocular vascular endothelial growth factor during aflibercept treatment of age-related macular degeneration. Am. J. Ophthalmol., 158(3): 532–536. 12.Ford J.A., Lois N., Royale P. et al. (2013) Current treatments in diabetic macular oedema: systematic review and meta-analysis. BMJ Open, 3(3): e002269. 13.Grover D., Li T.J., Chong C.C. (2008) Intravitreal steroids for macular edema in diabetes. Cochrane Database Syst Rev., 1: CD005656. 14.International Council of Ophthalmology (2014) ICO Guidelines for Diabetic Eye Care. Available at: http://www.icoph.org/enhancing_eyecare/international_clinical_guidelines.... Accessed February 7, 2015. 15.International Diabetes Federation — IDF (2015) IDF Diabetes Atlas, 7 ed. Brussels, Belgium, 144 р. 16.Korobelnik J.F., Do D.V., Schmidt-Erfurth U. et al. (2014) Intravitreal aflibercept for diabetic macular edema. Ophthalmology, 121(11): 2247–2254. 17.Luckie R., Leese G., McAlpine R. et al.; DARTS/MEMO Collaboration (2007) Fear of visual loss in patients with diabetes: results of the prevalence of diabetic eye disease in Tayside, Scotland (P-DETS) study. Diabet. Med., 24(10): 1086–1092. 18.Mitchell P., Wong T.Y.; Diabetic Macular Edema Treatment Guideline Working Group (2014) Management paradigms for diabetic macular edema. Am. J. Ophthalmol., 157(3): 505–513. 19.Muether P.S., Hermann M.M., Dr?ge K. et al. (2013) Long-term stability of vascular endothelial growth factor suppression time under ranibizumab treatment in age-related macular degeneration. Am. J. Ophthalmol., 156(5): 989–993. 20.Papadopoulos N., Martin J., Ruan Q. et al. (2012) Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis, 15(2): 171–185. 21.The Royal College of Ophthalmologists (2012) Diabetic Retinopathy Guidelines. Available at: https://www.rcophth.ac.uk/standards-publications-research/clinical-guide.... Accessed February 7, 2015. 22.Yau J.W., Rogers S.L., Kawasaki R. et al.; Meta-Analysis for Eye Disease (META-EYE) Study Group (2012) Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care, 35(3): 556–564.
|