J.ophthalmol.(Ukraine).2016;2:28-33.
|
https://doi.org/10.31288/oftalmolzh201622833 Assessment of the degree of diabetic neuropathy of the optic nerve following intravitreal interventions in proliferative diabetic retinopathy Elhage Emhamed Ali, Postgraduate Sudent A.A. Putienko, Dr Sc (Med) Filatov Institute of Eye Disease and Tissue Therapy Odessa (Ukraine) E-mail: alputienko@yandex.ru Purpose: To determine prospectively whether there is any relationship between a number of clinical factors and the degree of neuropathy of the optic nerve (ON) following intravitreal interventions in proliferative diabetic retinopathy (PDR). Materials and Methods: One hundred and five patients (105 eyes) with good anatomic results after undergoing vitrectomy were followed up and underwent visual evoked potential (VEP) testing at 2 months and 12 months. Results: At 2 months, neuropathy of the ON was observed in 56.1% of cases. Additionally, in 20.5% of these eyes, it was profound and characterized by intensely pale optic nerve head (ONH). At 12 months, the degree of neuropathy of the ON did not change. The functional activity of the visual system revealed by VEP tests (with 12-Hz flash, 1° and 0°15? checks) in study eyes was approximately 2-fold lower than normal values. Additionally, at both follow-up time points, the changes in VEP indices in eyes operated on for macular traction detachment were evidence of statistically significantly more profound degenerative processes in the nerve tissue than in eyes operated on for vitreous hemorrhage only. Conclusions: Progressive proliferative diabetic retinopathy is accompanied by a progressive neurodegenerative process in the retina and in the optic nerve, resulting in the development of a profound neuropathy of the optic nerve. The findings advocate the need for intensive neuroprotective therapy as well for as early as possible intravitreal intervention in this category of patients.
Key words: proliferative diabetic retinopathy, intravitreal interventions, optic nerve head neuropathy, visual evoked potential Introduction Proliferative diabetic retinopathy (PDR) is the leading cause of blindness in the industrialized European countries and in the USA [1]. Intravitreal surgeries are the main treatment for complications of PDR (such as hemorrhage, epiretinal membranes, tractional retinal detachment), and can contribute to stabilization of proliferative retinopathy and help to save visual function to some extent. The current view on the pathogenesis of ocular disease in diabetes mellitus (DM) is that neurodegenerative retinal changes precede vascular alterations and, as diabetic retinopathy evolves, they become more progressive [2, 3]. The disease is known to damage intraretinal layers initially, and later the external retina and the complete visual pathway become involved in the pathological process [3, 4]. Numerous studies have been published on the investigation of the degree of neuropathy of the optic nerve (ON) in both medically treated and non-treated patients with different stages of diabetic retinopathy, types, duration and degree of control of DM, as well as with juvenile DM [5-9]. However, to the best of our knowledge, no data have been reported on the influence of post-vitrectomy stabilization of the proliferative process on the course of neuropathy of the ON in PDR. The purpose of the study was to determine whether there is any relationship between a number of clinical factors and the degree of neuropathy of the ON following intravitreal interventions in PDR. Materials and Methods One hundred and five patients (105 eyes) with PDR who had undergone intravitreal interventions were included into this prospective study. The inclusion criteria were good anatomic outcome and transparent optical media. Patients underwent clinical assessment of neuropathy of the ON and visual evoked potential (VEP) testing at 2 months and 12 months following surgery. There were 46 men and 59 women, of whom, 22 subjects (21%) and 83 subjects (79.0%) had type I and type II diabetes mellitus (DM), respectively. Mean age was 53.8 years (SD, 14.4 years), and mean diabetes duration was 16 years (SD, 8.3 years). The numbers (percentages) of patients with adequately and sub-adequately controlled DM were 66 (62.9%) and 39 (37.14%), respectively. The level of diabetes control was assessed using daily variations in blood glucose level, and presence or absence of glucose and protein in urine. Forty two patients (40.0%) were hypertensive. The interval from the first sign of proliferative neuropathy till examination varied from 6 months to 32 months with mean value of 18.8 months (SD, 8.9 months). Nineteen eyes (18.1%) were pseudophakic, 63 eyes (60.0%) had incipient cataracts, and the rest of them had clear lenses. The intraocular pressure was within the normal range in all eyes. In 84 (80.0%) eyes, vitrectomy was performed after the patient’s neovascularization had proved unresponsive to panretinal laser coagulation. Indications for vitrectomy were vitreous hemorrhage without epiretinal membranes in 48 (45.7%) eyes (VI_VH_ONLY group), partial or total vitreous hemorrhage in the presence of epiretinal membranes and tractional retinal detachment involving the macula in 47 (44.8%) eyes (VI_VH+EM+MTD group), and combined traction and rhegmatogenous detachment in 10 (9.5%) eyes (VI_TRD+RRD group). Intravitreal interventions were performed using a standard technique. In brief, a core vitrectomy was followed by removal of epiretinal membranes (with removal of all islands of fibrovascular tissue), if any. Then, fluid air exchange for retinal folds was performed, and panretinal coagulation and coagulation of retinal breaks with endodiode laser were performed, if required. Among eyes of the VI_VH_ONLY group, surgeries were completed with intraocular tamponade using sterile air, 10% perfluoropropane and 20% perfluoropropane in 12 (25.0%) eyes, 16 (33.3%) eyes, and 20 (41.7%) eyes, respectively. The vast majority of surgeries in eyes of the VI_VH+EM+MTD group were completed with 20% perfluoropropane tamponade (38 (80.9%) eyes), and only 9 (19.1%) of them were completed with 10% perfluoropropane tamponade. 20% perfluoropropane tamponade was used in all eyes of the VI_TRD+RRD group. Initial visual acuity varied from light perception to 0.6 and, in the majority (89 (15.2%)) of eyes was lower than 0.1. Neuropathy of the ON was assessed clinically as follows: 0, pale pink optic nerve head (ONH); 1, moderately pale ONH; 2, intensely pale ONH. A RETIScan System (Roland Consult, Wiesbaden, Germany) was used to perform VEP tests according to the ISCEV Standards [10], with flash VEPs elicited by 2-Hz and 12-Hz flashes, and pattern VEPs elicited by checkerboard stimuli with large 1° and small 0.25° checks. All statistical analyses were performed by STATISTICA 9 software (Statsoft, Tulsa, OK). Student's t-test and Chi-square test were used for comparison of variables as appropriate. A P-value less than or equal to 0.05 was considered to be statistically significant. Results At month 2 after intravitreal intervention, the ONH was found to be pale pink (0 points), moderately pink (1 point), and intensely pale (2 points) (a) in 28 (58.3%) eyes, 12 (25.0%) eyes and 8 (16.7%) eyes, respectively, of the VI_VH_ONLY group; (b) in 14 (29.8%) eyes, 21 (44.7%) eyes and 12 (25.5%) eyes, respectively, of the VI_VH+EM+MTD group; and (c) in 4 (40.0%) eyes, 4 (40.0%) eyes and 2 (20.0%) eyes, respectively, of the VI_TRD+RRD group. The percentage of postoperative eyes with abnormally colored ONH in the VI_VH_ONLY group was found (a) to be statistically significantly lower than that in the VI_VH+EM+MTD group (?2 = 7.85, р = 0.005), and (b) to be not statistically significantly different from that in the VI_TRD+RRD group (?2 = 1.28, р = 0.289). Additionally, no statistically significant difference in the status of ONH was found between the VI_VH+EM+MTD group and the VI_TRD+RRD group (?2 = 0.40, р = 0.528). At month 12 after intravitreal intervention, no intragroup changes in the degree of neuropathy of the ON were found in the VI_VH_ONLY group and the VI_TRD+RRD group, and the number of postoperative eyes with normally colored ONH in the VI_VH+EM+MTD group decreased from 14 to 12, when compared to the previous time point. Consequently, at month 12, the percentage of postoperative eyes with abnormally colored ONH in the VI_VH_ONLY group was found again to be statistically significantly lower than that in the VI_VH+EM+MTD group (?2 = 10.48, р = 0.001). Therefore, in eyes of PDR patients displaying more severe proliferative changes and developing more mature epiretinal tissue preoperatively, the postoperative degree of neuropathy of the ON was statistically significantly higher. No statistically significant progress of neuropathy of the ON within 12 months of the follow-up was registered. However, it is noteworthy, that some progress of neuropathy of the ON was found in the eyes of the VI_VH+EM+MTD group, which was supported by a reduction in the number of eyes with normally colored ONH over time in this group. Table 1 presents the results of flash VEP tests (with flash VEPs elicited by a 2-Hz flash) performed in PDR patients at 2 months and 12 months after intravitreal interventions.
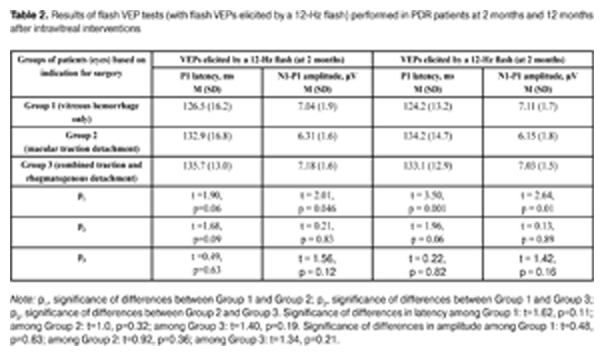
The data presented in Table 1 show that both at 2 months and 12 months after intravitreal interventions, no statistically significant intergroup differences in P2 amplitude and latency were observed. Additionally, among all the groups, flash VEPs elicited by a 2-Hz flash had within-the-norm latencies at both follow-up time points, with amplitudes 1.19 times lower and 1.18 times lower than normal levels at 2 months and 12 months, respectively. This is the evidence that patients had no severe alterations in the visual system in general (and in the visual pathways from the retina to the visual cortex in particular). Table 2 presents the results of flash VEP tests (with a 12-Hz flash) performed in PDR patients at 2 months and 12 months after intravitreal interventions. At 2 months after intravitreal interventions, no statistically significant intergroup difference in P1 latency was observed, with P1 amplitude in eyes operated on for macular traction detachment being statistically significantly lower than that in eyes operated on for vitreous hemorrhage only. Additionally, no statistically significant differences in P1 amplitude were observed (a) between eyes of the VI_VH_ONLY group and eyes of the VI_TRD+RRD group, and (b) between eyes of the VI_VH+EM+MTD group and those of the VI_TRD+RRD group. At 12 months after intravitreal interventions, P1 latency and P1 amplitude in eyes of the VI_VH+EM+MTD group were found to be statistically significantly increased and statistically significantly decreased, respectively, compared to eyes of the VI_VH_ONLY group. Additionally, no statistically significant differences flash VEP indices (with a 12-Hz flash) was observed (a) between eyes of the VI_VH_ONLY group and eyes of the VI_TRD+RRD group, and (b) between eyes of the VI_VH+EM+MTD group and those of the VI_TRD+RRD group. The study eyes in all the groups were found to experience no statistically significant changes over time (from 2 months to 12 months post surgery) in flash VEP indices (with a 12-Hz flash). It is noteworthy that, at 2 months time point, mean P1 latency was 1.32 times higher and mean P1 amplitude was 1.46 times lower, whereas, at 12 months time point, they were 1.31 times higher and 1.48 times lower, respectively, than normal levels, this being evidence of reduced functional activity of the visual system in patients with PDR following intravitreal interventions. The intragroup analysis of the indices revealed that eyes operated on for vitreous hemorrhage only experienced some improvement in VEP P1 latency and amplitude, whereas eyes of two other groups experienced some deterioration in these indices between 2 months and 12 months. Therefore, good anatomic outcome and stabilization of proliferative retinopathy achieved in eyes operated on for macular traction detachment and for combined traction and rhegmatogenous detachment (with these eyes having a more pronounced neuropathy of the optic nerve) was accompanied by progressive degenerative changes in the nerve tissue of the visual pathway.
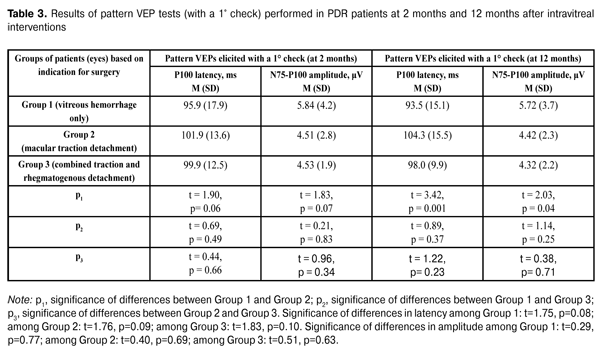
Table 3 presents the results of pattern VEP tests (with a 1° check) performed in PDR patients at 2 months and 12 months after intravitreal interventions. At 2 months after intravitreal interventions, no statistically significant intergroup difference in P1 latency was observed, with P1 amplitude in eyes operated on for macular traction detachment being statistically significantly lower than that in eyes operated on for vitreous hemorrhage only. At 12 months after intravitreal interventions, P100 latency was statistically significantly higher and P100 amplitude was statistically significantly lower in eyes operated on for macular traction detachment compared to those in eyes operated on for vitreous hemorrhage only. No statistically significant differences in P100 amplitude was observed (a) between eyes of the VI_VH_ONLY group and eyes of the VI_TRD+RRD group, and (b) between eyes of the VI_VH+EM+MTD group and those of the VI_TRD+RRD group. There were no statistically significant intragroup changes in indices of pattern VEP tests (with a 1° check) between 2 months and 12 months. Additionally, among all the groups, pattern VEPs elicited by a 1° check had within-the-norm P100 latencies at both follow-up time points, with the P100 amplitudes being approximately 2-fold lower than normal values. Moreover, pattern VEPs elicited by a 1° check demonstrated a progressive reduction in the functional activity of the nerve tissue of the visual system in eyes with initially more pronounced proliferative and, correspondingly, neurodegenerative alterations.
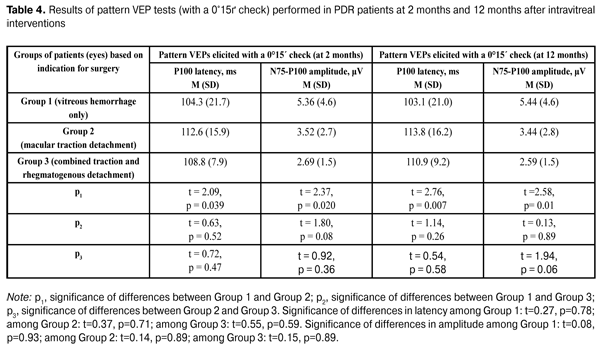
Table 4 presents the results of pattern VEP tests (with a 0°15? check) performed in PDR patients at 2 months and 12 months after intravitreal interventions. The data presented in Table 4 show that both at 2 months and 12 months, P100 latency was statistically significantly higher and P100 amplitude was statistically significantly lower in eyes operated on for macular traction detachment compared to those in eyes operated on for vitreous hemorrhage only. In these tests (similar to flash VEP and pattern VEP tests with a 1° check), no statistically significant differences in P100 latency and amplitude was observed (a) between eyes of the VI_VH_ONLY group and eyes of the VI_TRD+RRD group, and (b) between eyes of the VI_VH+EM+MTD group and those of the VI_TRD+RRD group. There were no intragroup changes in indices of pattern VEP tests (with a 0°15? check) between 2 months and 12 months. It is noteworthy that, at both time points, mean P100 amplitude was significantly (2.6 times at average) lower than normal levels, whereas mean P100 latency was within the normal range. Consequently, a profound reduction in the response of the region of visual cortex that responds to the fovea occurred in eyes with PDR following intravitreal interventions, with the most apparent reduction being observed in eyes operated on for macular traction detachment.
The number of eyes with a visual acuity (VA) of 0.1 or better statistically significantly increased in the VI_VH_ONLY group and the VI_VH+EM+MTD group at both follow-up time points compared to baseline. No statistically significantly increase in the number of eyes with a VA of 0.1 or better was observed in the VI_TRD+RRD group. Table 5 presents the data on the eyes with this VA. It is noteworthy that, baseline VA was statistically significantly lower in eyes with vitreous hemorrhage than in those with macular traction detachment and with combined traction and rhegmatogenous detachment, due to reduced transparency of the optic media. Additionally, at both time points, a statistically significantly higher VA was found in eyes operated on for vitreous hemorrhage only than in those operated on for other indications. Consequently, in patients with initially profound proliferative changes (in whom a more apparent neuropathy of the ON was observed), a statistically significantly lower VA was noted. Conclusion In conclusion, at 2 months following an intravitreal intervention for complications of PDR, neuropathy of the ON was observed in 56.1% of the eyes with a good anatomic outcome. Additionally, in 20.5% of these eyes, it was profound and characterized by intensely pale ONH. At 12 months, the degree of ONH neuropathy did not change. In eyes with profound proliferative alterations (namely, macular traction detachment) at baseline, the frequency of neuropathy of the ON at follow-up time points was statistically significantly higher than that in eyes having no epiretinal tissue (those operated on for vitreous hemorrhage only). The mean level of functional activity of the visual system revealed by VEP tests (with 12-Hz flash, 1° and 0°15? checks) in eyes that had undergone intravitreal intervention in PDR was approximately 2-fold lower than normal values. Additionally, at both follow-up time points, the changes in VEP indices in eyes operated on for macular traction detachment were evidence of statistically significantly more profound degenerative processes in the nerve tissue than in eyes operated on for vitreous hemorrhage only. Progressive proliferative diabetic retinopathy is accompanied by a progressive neurodegenerative process in the retina and in the optic nerve, resulting in the development of a profound neuropathy of the ON. In patients with initially profound proliferative changes, in spite of stabilization of proliferative disease following successful intravitreal surgery, progressive degeneration of the nerve tissue of the visual system was observed within a follow-up year. The findings advocate the need for intensive neuroprotective therapy as well for as early as possible intravitreal intervention in this category of patients. References
|