J.ophthalmol.(Ukraine).2016;1:43-47.
|
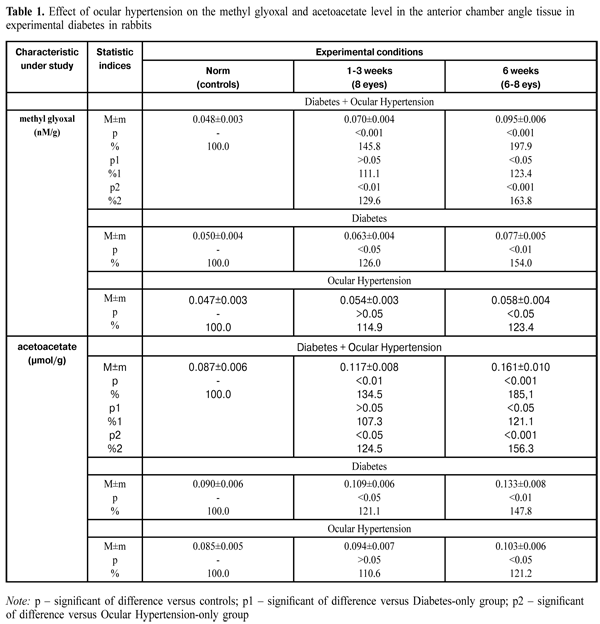
https://doi.org/10.31288/oftalmolzh201614347 Effect of ocular hypertension on the levels of glycation products in anterior eyes tissues in streptozotocin-induced diabetes V.R.Yurevich, Cand. Sc. (Med) Danylo Halytsky Lviv National Medical University Lviv, Ukraine E-mail: yurevych@yahoo.com Introduction Glaucoma is an important cause of irreversible blindness in the world. Primary open-angle glaucoma (POAG) is considered to be the most widespread type. Age, myopia, central thickness of the cornea and ocular hypertension are risk factors for POAG [1]. The presence of diabetes mellitus (DM) has been reported to be another possible risk factor for POAG occurrence. However, the mechanism of the effect of DM on the development of POAG is understudied [2]. One of the causes of increased intraocular pressure (IOP) and POAG development in DM patients is morphological changes in the eye drainage system tissue which are related to disorders in protein and lipid metabolism. These changes have been reported to be an immediate cause of ocular hydrodynamics disorders since aqueous humor outflow is impeded in DM patients [3, 4] The recent investigations have revealed that, in both glaucoma and diabetes, significant disorders are noted at the level of highly reactive compounds in the retina, such as methyl glyoxal and acetoacetate [5, 6, 7, 8, 9, 10]. We believe that studying processes causing alterations at the level of toxic products of glycosylation in glaucoma associated with diabetes mellitus is of special interest. The purpose of the present study is to determine the concentration of glycation products in anterior chamber angle tissues in a model simulating ocular hypertension in streptozotocin-induced diabetes. Materials and Methods 32 Chinchilla adult male rabbits (weighted 2.5-3.2 kg) were used in the experimental study. All work with animals followed Guiding Principles for Biomedical Research Involving Animals issued by Council for the International Organizations of Medical Sciences (2012). The animals were divided into four groups: group 1 (controls; 10 rabbits), group 2 (diabetes and ocular hypertension; 8 rabbits), group 3 (diabetes-only; 7 rabbits) and group 4 (ocular hypertension-only; 7 rabbits). All groups were subdivided into two groups on dependence on the study time points: subgroup 1 (three weeks) and subgroup 2 (six weeks). Diabetes was induced by a single intravenous injection of streptozotocin (SZT) (65 mg per 1 kg body weight) [11]. At Day 2 after SZT injection, the animals of groups 2 and 4 were induced ocular hypertension by a 0.2% methylcellulose injection into the ocular anterior chamber. Immediately after the injection, the rabbits were examined biomicroscopically to assess injection-related trauma [12]. Before modelling ocular hypertension, the animals were anesthetized generally by ketamine (50 mg/kg), and locally by 0.5% procaine hydrochloride instillations. Each animal was examined ophthalmologically and IOP was measured before and during the experiment. Tonometry was performed every few hours. The animals were euthanized by a pentobarbital sodium (100 mg/kg) injection administered vie the marginal ear vien. Methyl glyoxal, acetoacetate and carbonyl groups of protein were determined in the tissues of anterior chamber angle. The technique to determine the methyl glyoxal level is based on the spectrophotometric assay of S- lactoylglutathione; the latter is a product of reaction of methyl glyoxal with glutathione and has an absorption maximum at 240nm. The coefficient of variation of the technique is 5.1% [13]. The technique to determine the acetoacetate level is based on the spectrophotometric assay of a decreased concentration of reduced NAD in a result of reduction reaction of acetoacetate by 3-hydroxybutyrate dehydrogenase. The coefficient of variation of the technique is 6% [13]. The technique to determine the carbonyl groups is based on the spectrophotometric assay of chromogenic product of protein carbonyl derivatization with 2 4-dinitrophenylhydrazine. The coefficient of variation of the technique is 4.7% [14, 15]. The data obtained were statistically processed with SPSS 11.0 software [16]. Results and Discussion The Table 1 presents the data on the effect of ocular hypertension on the methyl glyoxal and acetoacetate levels in the anterior chamber angle tissue in experimental diabetes in rabbits.
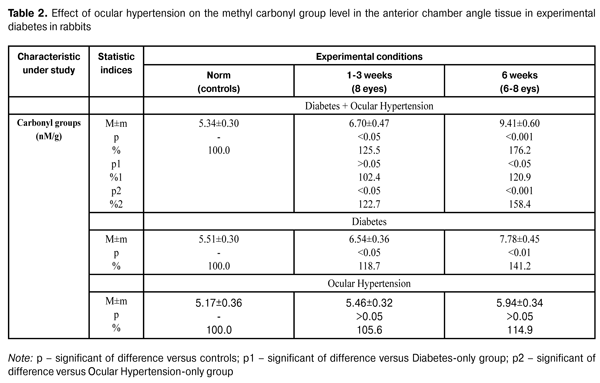
The table shows that the methyl glyoxal level in the anterior chamber angle tissue in rabbits with diabetes and ocular hypertension increased to 0.070±0.004 nM/g (145.8%) (р<0.001) and to 0.095±0.006 nM/g (197.9%) (р<0.001) at time points of 1-3 weeks and 6 weeks, respectively, as compared to the norm 0.048±0.003 nM/g (р<0.001). The measurement of the methyl glyoxal level in the anterior chamber angle tissue in rabbits with ocular hypertension revealed its increase to 0.054±0.003 nM/g (114.9%) and to 0.058±0.004 nM/g (123.4%) at time points of 1-3 weeks and 6 weeks, respectively, as compared to controls 0.047±0.003 nM/g (р<0.05). Comparing the data of these two groups (diabetes and ocular hypertension/ocular hypertension), it can be seen that the increase in the methyl glyoxal level in the anterior chamber angle tissue was higher in the former than in the latter. Thus, the difference was 29.6% and 63.8% at time points of 1-3 weeks and 6 weeks, respectively (р<0.001). It should be noted that the methyl glyoxal level in the anterior chamber angle tissue in rabbits with diabetes increased to 0.063±0.004 nM/g (126%) (р<0.05) and to 0.077±0.005 nM/g (154%) at time points of 1-3 weeks and 6 weeks, respectively, as compared to the controls 0.050±0.004 nM/g (р<0.01). Herewith, this statistic index of the group with diabetes and ocular hypertension was higher than that in the diabetes-only group. Thus, the increase was equal to 11.1% and 23.4% at time points of 1-3 weeks and 6 weeks, respectively, (р<0.05). As can be seen in the Table 1, the acetoacetate level in the anterior chamber angle tissue in rabbits with diabetes and ocular hypertension was increased and equaled 134.5% (0.117±0.008 µmol/g) (р<0.01) and 185.1% (0.162±0.010 µmol/g) at the first time point and the second time point, respectively, as compared to the norm 0.087±0.006 µmol/g (р<0.001). In rabbits with a model simulating ocular hypertension, the acetoacetate level in the anterior chamber angle tissue increased to 0.094±0.007 µmol/g (110.6%) and to 0.103±0.006 µmol/g (121.2%) comparing to the controls (0.085±0.005) µmol/g (р<0.05). It should be pointed, that within the study period, the acetoacetate level in the anterior chamber angle tissue was increased in diabetic rabbits with ocular hypertension as compared to that in animals in ocular hypertension-only group. Thus, the difference was equal to 24.5% (р<0.05) and 56.3% (р<0.001) at the first time point and the second time point, respectively. The acetoacetate level in the anterior chamber angle tissue in diabetic rabbits with ocular hypertension was increased and equaled 134.5% (0.117±0.008 µmol/g) (р<0.01) and 185.1% (0.162±0.010 µmol/g) at the first time point and the second time point, respectively, as compared to the norm 0.087±0.006 µmol/g (р<0.001). The acetoacetate level in the anterior chamber angle tissue in rabbits with diabetes-only increased to 121.1% (0.109±0.006 µmol/g) (р<0.05) and to 147.8% (0.133±0.008 µmol/g) at the first time point and the second time point, respectively, as compared to the controls 0.090±0.006 µmol/g (р<0.01). It should be noted that the increase in the acetoacetate level in the anterior chamber angle tissue in diabetic rabbits with ocular hypertension was higher than that in diabetes-only rabbits. Thus, the difference equaled 7.3% and 21.1% at the first time point and the second time point, respectively. The Table 2 demonstrates the data on the effect of ocular hypertension on the carbonyl groups in the anterior chamber angle tissue in experimental diabetes in rabbits.
The carbonyl group level in the anterior chamber angle tissue in diabetic rabbits with ocular hypertension increased to 125.5%, i.g. (6.70±0.47) nM/g (р<0.05) and to 176.2%, i.g. (9.41±0.60) nM/g as compared to the norm (5.34±0.30) nM/g (р<0.001). The carbonyl group level in the anterior chamber angle tissue in rabbits with ocular hypertension-only increased to 105.6%, i.g. (5.46±0.32) nM/g and to 1114.9%, i.g. (5.94±0.34) nM/g at time points of 1-3 weeks and 6 weeks, respectively, as compared to the controls (5.17±0.36). Herewith, the increase in the carbonyl group level in the anterior chamber angle tissue was high in rabbits in diabetes and ocular hypertension group than that in ocular hypertension-only group. Thus, the difference was equal to 22.7% (р<0.05) and 58.4% (р<0.001) at time points of 1-3 weeks and 6 weeks, respectively. In diabetes-only group, the carbonyl group level in the anterior chamber angle tissue increased to 118.7%, i.g (6.54±0.36) nM/g (р<0.05) and to 141.2%, i.g (7.78±0.45) nM/g at time points of 1-3 weeks and 6 weeks, respectively, compared to the controls (5.51±0.30) nM/g (р<0.01). It should be noted, that within the study period, the carbonyl group level in the anterior chamber angle tissue was increased in rabbits with diabetes and ocular hypertension as compared to that in rabbits in diabetes-only group. Thus, the difference was equal to 2.4% and 20.9% (р<0.05) at time points of 1-3 weeks and 6 weeks, respectively. Thus, as can be seen from the data obtained in this study, the development of ocular hypertension in the streptozotocin-induced diabetic rabbits results in the increase in methyl glyoxal and acetoacetate levels and in the protein carbonyl group concentration in the anterior chamber angle tissue. We suppose that the state of oxidative stress in the anterior segment of the eye under the conditions of increased IOP plays the main role in the increased rate of glycation in the anterior chamber angle tissue in ocular hypertension, which is mostly apparent in the streptozotocin-induced diabetic rabbits. This is confirmed by studies on peroxidation in the aqueous humor, tear fluid and anterior chamber angle tissue in glaucoma [17, 3, 1, 18]. In general, the data obtained give an insight into pathogenic mechanism underlying the development of glaucoma in diabetes patients. Conclusions 1. Ocular hypertension in rabbits with diabetes results in a jump in the hydroxy aldehyde level in the anterior chamber angle tissue. In such conditions, the methyl glyoxal level is significantly higher than that in animals with models simulating separately either ocular hypertension or diabetes. 2. A significant increase in the carbonyl group level was revealed in diabetic animals with ocular hypertension; this is evidence that the damage of proteins in drainage system of the eye is more severe in concomitant diseases.
References 1. Serdyuk VN. Investigation of the level of products of proteins’ and lipids’ oxidative damage in the tear fluid and chamber moisture in POAG patients. Problemy ekologichnoi ta medychnoi genetiki ta klinichnoi imunologii. 2011;6(51):168-77. 2. Astakhov YuS, Krylova IS, Shadrichev FE. [Is diabetes mellitus a risk factor for primary open-angle glaucoma?] Russkii med. Zhurn. 2005;3(2):56-9. In Russian. 3. Kashintseva LT. [The role of metabolic disorders in the pathogenesis of glaucoma in the insular system disorder]. Oftalmol Zh. 1970;7:531-6. In Russian. 4. Adeoti CO, Isawumi MA, Ashaye AO. The anterior segment of the eye in diabetes. Clinical Ophthalmology. 2012;6:667–1. 5. Bierhaus A, Fleming T, Stoyanov S. Methylglyoxal modification of Nav1.8 facilitates nociceptive neuron firing and causes hyperalgesia in diabetic neuropathy. Nat. Med. 2012;18:926–33. 6. Duran-Jimenez B, Dobler D, Moffatt S. Advanced glycation end products in extracellular matrix proteins contribute to the failure of sensory nerve regeneration in diabetes. Diabetes. 2009;58:2893–903. 7. Matafome P, Sena C. Methylglyoxal, obesity, and diabetes. Endocrine. 2013;43(3):472-84. 8. Rabbani N. Critical role of methylglyoxal and glyoxalase 1 in diabetic nephropathy. Diabetes. 2014;63:50-2. 9. Riboulet-Chavey A, Pierron A, Durand I. Methylglyoxal impairs the insulin signaling pathways independently of the formation of intracellular reactive oxygen species. Diabetes. 2006;5:1289-99. 10. Sienkiewicz AE, Rosenberg BN, Edwards G. Aberrant glycosylation in the human trabecular meshwork. Proteomics Clin. Appl. 2014; 8(3-4):130-42. 11. Wang J, Wan R, Mo Y. Creating a long-term diabetic rabbit model. Exp. Diabetes Res. 2010;6:1-10. 12. Zhu MD, Cai FY. Development of experimental chronic intraocular hypertension in the rabbit. Australian and New Zeland J. Ophthalmol. 1992;20:225-34. 13. Bergmeyer HV. Metoden der enzymatischen Analyse. Berlin; 1986. 2220 p. 14. Davanand CD, Vegi PK. Protein carbonyl content as a stable oxidative stress marker in type II diabetes. Int. J. Biol. Med. Res. 2012;3:2362-5. 15. Odetti P, Garibaldi S, Noberasco G. Levels of carbonyl groups in plasma proteins of type 2 diabetes mellitus subjects. Acta Diabetol. 1999;36(4):179-83. 16. Rebrova OJu. [Statistical analysis of medical data. The use of STATISTICA software]. Moscow: MediaSfera; 2002. 295 p. In Russian. 17. Kashintseva LT. [The pathogenic role of changes in drainage system of the eye in the development of glaucoma in diabetes patients]. Oftalmol Zh. 1971;5:381-6. In Russian. 18. Yurevich VR. Effect of ocular hypertension on the levels of lipid peroxidation products in anterior eye tissues in experimental diabetes. J.ophthalmol.(Ukraine).2015;4:38-43.
|