J.ophthalmol.(Ukraine).2016;1:36-42.
|
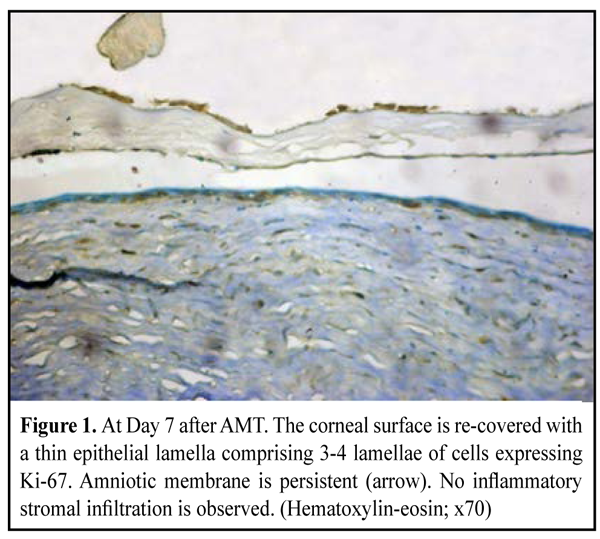
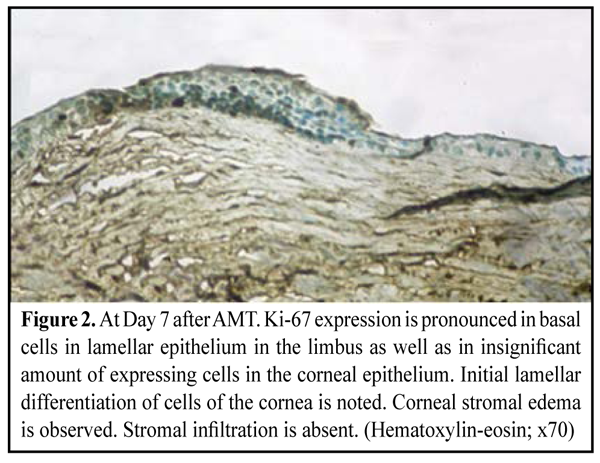
https://doi.org/10.31288/oftalmolzh201613642 Corneal inflammation and proliferative activity of cells of anterior epithelium in modeling bacterial keratitis and using amniotic membrane fixed with different techniques E.V. Sereda V.V. Vit, Dr. Sc. (Med), Prof G.I. Drozhzhina, Dr. Sc. (Med), Prof T.B. Gaidamaka, Dr. Sc. (Med) Filatov Institute of Eye Diseases and Tissue Therapy Odessa, Ukraine E-mail: evsereda08@gmail.com Introduction. Molecular mechanisms of the effect of amniotic membrane (AM) on the inflammation and recover of the cornea have been investigated using various biological markers. Purpose. To determine the dependence between corneal inflammation and proliferative activity of anterior epithelium cells studying the expressions of Ki-67, CD-68, ММР-9 in a model simulating bacterial keratitis and when using amniotic membrane fixed with different techniques. Material and Methods. 60 chinchilla rabbits (60 eyes) were involved into the experimental study. The animals were induced moderate bacterial keratitis. In two weeks, the rabbits were performed AM transplantation; two techniques were used: an inlay technique with AM secured to the cornea with eight interrupted 10/00 nylon sutures, and an onlay technique with AM sutured to episclera using eight interrupted 8/00 silk sutures. Results. The study revealed the presence of negative correlation between a proliferative activity of anterior corneal epithelial cells and a degree of inflammatory infiltration of the corneal stroma as evidenced by studying such markers of corneal repair as Ki-67, CD 68 and MMP-9. If inflammation occurs, the proliferative activity of anterior epitehelium cells is reduced that significantly decreases epithelization rates and following lamellar cell differentiation. Conclusions. Inflammation and epithelization rate reduction occurs more often when AM is sutured to the corneal surface, i.g. in group of experimental animals, in which the inlay technique of AMT was used. Key words:bacterial keratitis, amniotic membrane, experiment, biological markers, repair Introduction Human amniotic membrane (AM) has taken a strong place in ocular surface reconstructive surgery for its antibacterial, antiangiogenic, anti-inflammatory and antifibroplastic features [1, 2, 3, 4]. Thus, AM may play an important role in the treatment of infectious keratitis. The most of experimental and clinical studies have been on the influence of amniotic membrane transplantation (AMT) on the course of herpetic and neurotrophic keratitis as well as eye burns. A. Heiligenhaus et al. have studied healing and neovascularization of the cornea after ATM in experimental ulcerative herpetic keratitis. The expression of matrix metalloproteinase-9 has been determined [5]. D. Вauer et al have investigated the effect of AM on T-cell immune response in the cornea of mice with herpetic stromal keratitis and revealed that AM induces apoptosis in T lymphocytes [6]. There are also single papers on the effect of AMT on the inflammatory process in the cornea in bacterial keratitis [7, 8, 9]. At the present time molecular mechanisms of AM effect on the course of inflammation and healing of the cornea have been studied using different biological markers, in particular IL-6, IL-10, a tumor necrosis factor (TNF-?), galectin 1, proliferating cell nuclear antigen (PSNA) and matrix metalloproteinase (MMP) [10, 11, 5, 12]. We chose biological markers Ki-67, CD 68 and MMP-9 to investigate the effect of AMT on the course of inflammatory process in the cornea in bacterial keratitis. Ki-67 is known as a marker of epithelial cell proliferation. The expression of this protein occurs during G1 phase, accumulates during the cell cycle and rapidly decreases during the mitotic phase [13]. Thus, the expression of Ki-67 identifies cells in all cell cycle phases except for non-cycling state G0 [11, 14]. Amount and appearance terms of Ki-67 positive cells estimate corneal epithelization process [15, 16]. A CD68 marker is a glycoprotein which is expressed on the surface of monocytes and macrophages and is used as a marker of macrophages. It is known that AMT can remarkably decrease corneal inflammation and reduce the risk of complications such as descemetocele and perforated ulcer [17, 18]. That's why determination of an expression level of CD 68 in the corneal stroma can assess the degree of corneal infiltration [19] and AM anti-inflammatory action [20]. MMP-9 is an enzyme gelatinase and takes part in remodeling of extracellular matrix. Although MMP is secreted by different cells including trophoblasts, osteoclasts, neutrophils and macrophages, the most its amount is secreted by polymorphonuclear leukocytes. MMP is a part of corneal response to injury and infection [21]. The role of increased expression of MMP-9 in potentiating the inflammation and degradation of the corneal tissue has been proved in a model simulating Candida albicans keratitis [22]. Heiligenhaus A. et al. have revealed the positive dependence of a decrease of corneal inflammation and neovascularization on the reduced expression of MMP-9 after AMT in a model simulating ulcerative herpetic keratitis [5]. Thus, we believe that studying a MMP-13 secretion level in a model of bacterial keratitis after AMT is of clinical and scientific interest. The purpose of the present paper is to determine a correlation between corneal inflammation and proliferative activity of cells of anterior epithelium on the basis of studying expressions of KI-67, CD-68, MMP-9 when using amniotic membrane with its different fixation techniques in a model simulating bacterial keratiitis. Material and Methods An experimental study was performed in 60 chinchilla rabbits (60 eyes) weighted 2.5-3.0 kg. The animals were housed at room temperature and fed with a standard laboratory diet. Surgical interventions were performed at the vivarium at the Filatov Institute of Eye Disease and Tissue Therapy under aseptic and antiseptic conditions. The experiment followed ethical standards provided by the European Treaty Series European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (Strasbourg, 1986), the First National Bioethics Congress of Ukraine (Kiev, 2001), and Law of Ukraine No.3447-IV On the Protection of Animals from Cruelty (Kiev, 2006). Moderate bacterial keratitis in rabbit was induced as we had described previously [23]. So, the cornea was prephined to two thirds of stromal depth and infected by two time instillation of 1ml bacterial suspension of Staphylococcus aureus (109 cell/mL) obtained from diseased. The instillations were followed by a subconjunctival injection of 0.1 mL diprospan [13]. At two weeks after modeling keratitis 60 eyes from 60 animals were subjected to AMT. To thaw the membrane, a cryoval was removed from the liquid nitrogen Dewar and placed into water bath with temperature of +38-40°С. In 30 experimental animals, an inlay technique was used and the amnion was sutured to the cornea with eight interrupted 10/00 nylon sutures, whilst in other 30 animals, an onlay technique was used with membrane sutured to episclera using eight interrupted 8/00 silk sutures. The eyelids of the animals were sewn together using two "U" sutures, leaving a narrow opening in medial angle to look through. The follow up period was 1 month. The rabbits were euthanized at 7, 14 and 30 days (10 animals at each time point) by a 1.0 cm3 air injection in marginal ear vein. Thereafter, corneal tissue was harvested to perform morphological study. Immunohistochemistry An indirect streptavidin-peroxidase method was used to investigate the expression of monoclonal antibodies (MAb) to CD68, Кi-67, and ММР-9. In brief, tissue sections were deparaffinated and endogenous peroxidase was blocked with 3% hydrogen peroxide solution; afterwards the sections were rinsed and non-specific proteins were blocked with 1% BSA. Sections were washed in PBS buffer, and primary antibodies were placed to antigens (DAKO, Denmark) for one hour. After washing in PBS buffer, second antibodies were placed. After another washing in PBS buffer, two drops of streptavidin and peroxidase complex were added with following incubation for 30 min. Finally, sections were washed again and AEC Chromogen solution was placed with following incubation for 5-20 minutes until brown color appeared. Results Day 7 after AMT Group of the experimental animals, in which a biological covering of AM (onlay technique) was used (30 eyes, 30 rabbits) Moderate apparent and moderate corneal stromal edema was observed in 16/30 eyes and 4/30 eyes, respectively. Corneal edema was absent in 10/30 eyes. Surrounding stromal edema was moderate apparent and intact in 12/30 eyes and 12/30 eyes, respectively. Complete lysis and partial lysis of the AM was found in 6 eyes and 14 eyes, respectively. The AM on the corneal surface remained persistent in10/30 eyes. Group of the experimental animals, in which lamellar AMT (inlay technique) was used (30 eyes, 30 rabbits) Corneal stromal edema was moderate apparent and moderate in 24/30 eyes and 6/30 eyes, respectively. The presence of moderate apparent surrounding stromal edema was observed in 16/30 eyes and diffuse edema was noted in 6/30 eyes. No surrounding stromal edema was found in 8/30 animals. No lysis, partial lysis and complete lysis was found in 14 eyes, 10 eyes and 6 eyes, respectively. Ki-67 expression. At day 7 after AMT, we didn’t observe the difference in KI-67 expression ratio in both groups. Histological study of the corneal tissue revealed stromal edema, accompanied by a diffuse and focal reduction in the number of keratocytes, in all the animals. Also, mucoid edema of stromal collagen lamellae in the deeper layers was observed. No apparent inflammatory changes were noted in the central cornea. Inflammatory infiltrates were observed near the limbus and around the suture. At the time point of 7 days, the wound was covered with a thin epithelium comprising 2-3 epithelial flattened cell lamellae with no lamellar differentiation. Herewith, Ki-67 was expressed in almost all flattened epithelial cells, and that was an evidence of high proliferative activity of the latter (Fig. 1). Proliferative activity was the most pronounced in basal epithelial cells of limbal area since basal epithelial cells are the source of cellular elements covering the wound corneal surface (Fig. 2). Despite corneal stromal edema, the expression of Ki-67 was also observed in keratocytes diffusively distributed in the stroma (Fig. 1, 2).
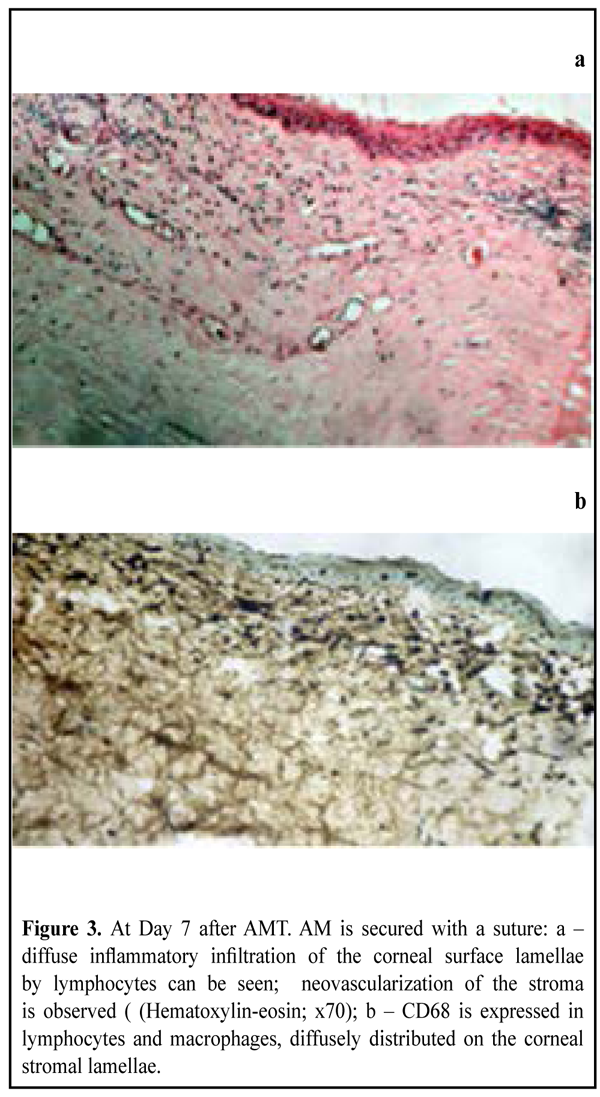
At the time point of 7 days, AM remnants could be found in most of the animals (Fig. 1). CD68 expression. Being a marker of monocytes, lymphocytes and macrophages, the expression of CD68 was observed on the corneal stromal surface, as well as in sheaths around blood vessels, with various differentiation degrees, and epithelial cells, in the animals underwent lamellar transplantation of AM. A positive reaction was also noted in macrophages diffusively distributed in the corneal stroma near the site of neovascularization. As evidenced by the absence of Ki-67 expression, proliferative activity of cells of anterior corneal epithelium was decreased or absent, even on the edges of epithelial defect. CD68 expression was observed in none of the animals with AM biological covering to the corneal surface and without sutures (Fig. 4). It should be noted that the corneal surface was covered with completely differentiated epithelium, and edema persisted in the stroma. An artificial reaction was found only in some keratocytes and on the surface of stromal collagen lamellae in the deeper corneal layers. No signs of inflammatory infiltration of the corneal stroma were revealed.
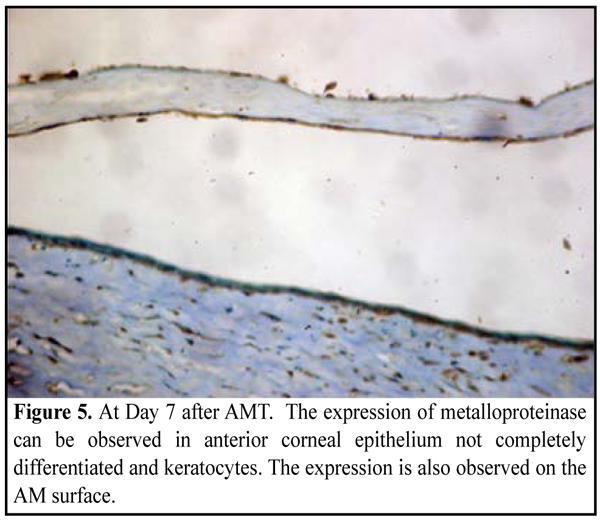
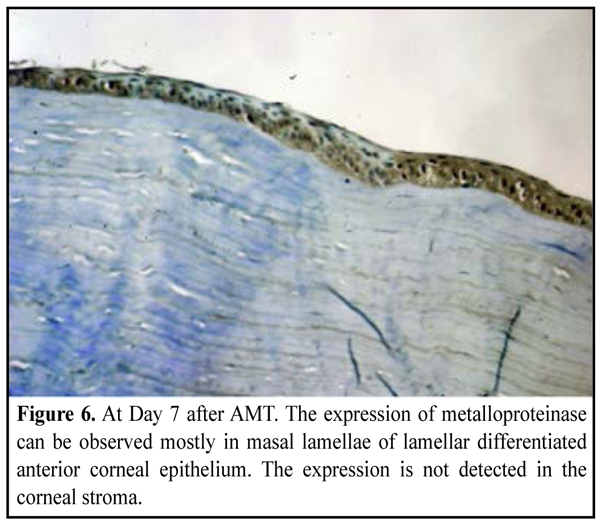
MMP-9 expression. Being a descriptor of inflammatory process and recovery of the corneal stroma, the expression of metalloproteinase was observed in cells of anterior epithelium not depending on the differentiation degree of the latter, as well as in stromal keratocytes (Fig. 5, 6), in most cases of both groups. Attention should be paid to a pronounced reaction in the cells of AM (Fig. 5). MMP-9 appeared to be expressed in both cells of anterior corneal epithelium and AM enzyme diffusion.
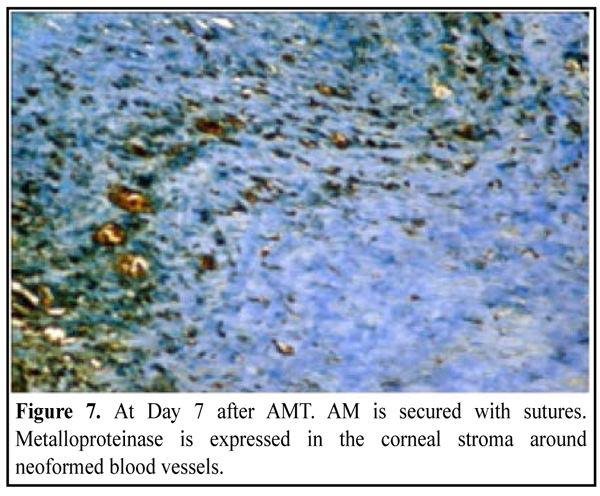
The most pronounced expression was revealed in the sites of neovascularization of the corneal stroma when suturing AM (the group with lamellar AMT); this was evidenced by the inflammatory reaction persisted and alterations of CT elements near neoformed blood vessels (Fig. 7).
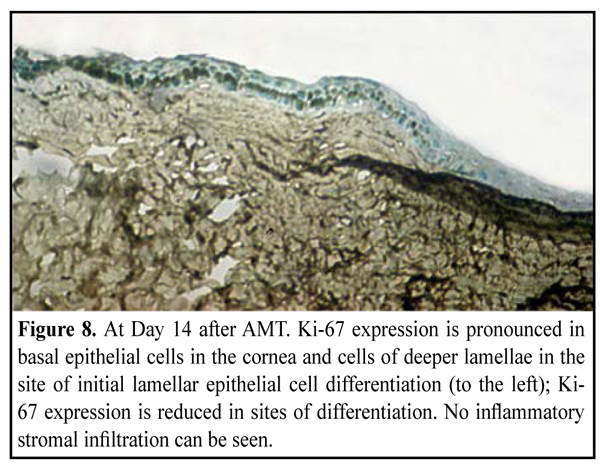
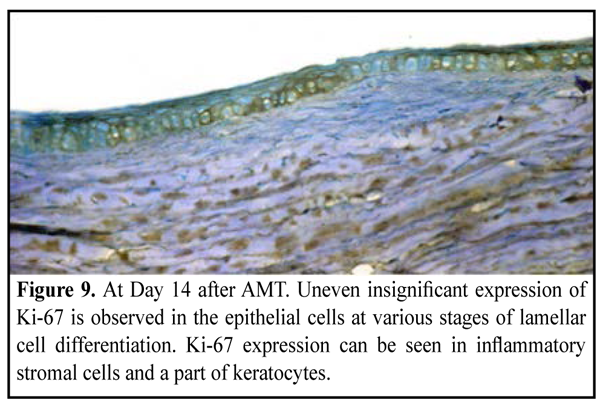
Day 14 after AMT Group of the experimental animals, in which a biological covering of AM (onlay technique) was used (20 eyes, 20 rabbits) Moderate apparent corneal stromal edema was observed in 12/20 eyes and moderate apparent surrounding stromal edema was in 4/20 eyes. Fluorescein staining revealed punctate infiltrates of the cornea in 2 eyes. No lysis, partial lysis and complete lysis was found in 2 eyes, 10 eyes and 8 eyes, respectively. Group of the experimental animals, in which lamellar AMT (inlay technique) was used (20 eyes, 20 rabbits) Moderate apparent and moderate corneal stroma edema was observed in 6/20 eyes and 4/20 eyes, respectively. Surrounding stromal edema was moderate in 8 eyes. Fluorescein staining revealed punctate infiltrates of the cornea in 6 eyes. No lysis, partial lysis and complete lysis was found in 4 eyes, 12 eyes and 4 eyes, respectively. Ki-67 expression. At the time point of 14 days, the corneal surface was completely re-covered with epithelial cells comprising 5-6 epithelial cell lamellae. Lamellar differentiation of epithelial cells was observed. Herewith, there were a reduced number of epithelial cells expressing Ki-67 or a complete absence of Ki-67 expression (Fig. 8, 9). In some cases, epithelization was absent at the suture sites. Inflammatory infiltrate was formed around the suture; and the former mostly comprised of lymphocytes expressing Ki-67.
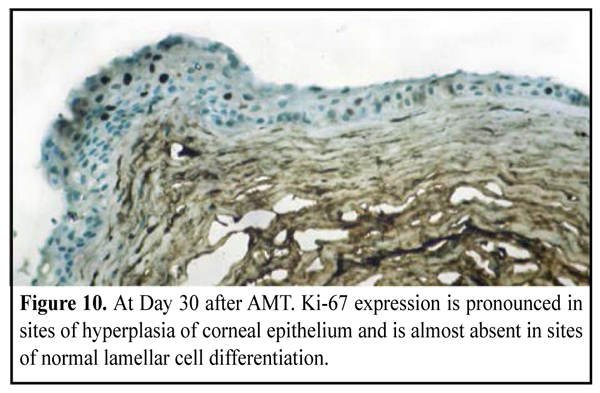
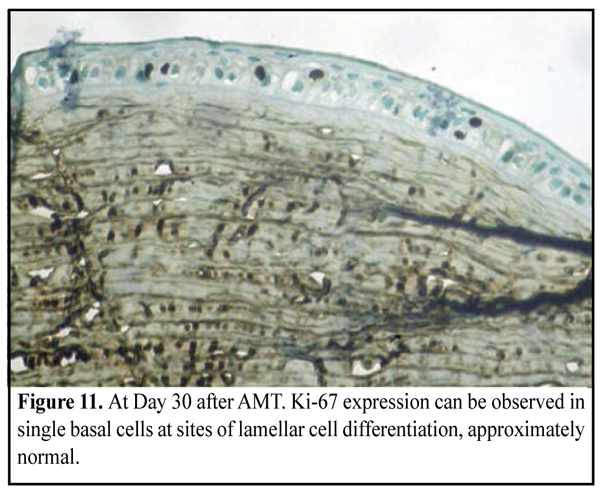
CD68 and ММР-9 expression. At Day 14, a positive reaction was absent in the corneal stroma, except for sites near the sutures in the animals undergone lamellar AMT. Day 30 after AMT Group of the experimental animals, in which a biological covering of AM (onlay technique) was used (10 eyes, 10 rabbits) Moderate apparent corneal stromal edema was observed in 4/10 eyes. Fluorescein staining test was negative and no corneal infiltration was observed in all animals. Partial lysis and complete lysis was found in 4 eyes and 6 eyes, respectively. Soft cloud-like corneal opacity was formed in 6 eyes. Group of the experimental animals, in which lamellar AMT (inlay technique) was used (10 eyes, 10 rabbits) Fluorescein staining revealed punctate infiltrates of the cornea in 4/10 eyes. The AM was persistent on the corneal surface in 4 eyes. Partial lysis and complete lysis was found in 4 eyes, and 2 eyes, respectively. Soft cloud-like and intensive corneal opacity was formed in 2 eyes and 4 eyes, respectively. In 4 eyes, corneal neovascularization was observed. Ki-67 expression. At the time point of 30 days, a histological study revealed complete lamellar differentiation of epithelial cells, in both groups. At the same time, the number of lamella and a degree of their differentiation was various at different sites. In such cases, Ki-67 expression was absent. A number of proliferating cells was decreased at sites of differentiation of epithelial cells which were approximately normal. At the same time, at the sites of focal hyperplasia of epithelium there was revealed a great number of cells, especially of surface epithelium lamellae, expressing Ki-67 (Fig. 10, 11).
CD68 and ММР-9 expression. At Day 30, a positive reaction was absent in the corneal stroma, except for sites near sutures in the animals undergone lamellar AMT. Conclusion Thus, at the time points of 14 and 30 days, complete epithelization of the cornea with lamellar differentiation of the cells was accompanied with reduction or complete absence of proliferative activity of epithelial cells, in both groups. Herewith, there were no inflammation signs except the site near AM fixation sutures in group with lamellar AMT. This was evidenced by the absence of the positive reaction in the corneal stroma when determining CD68 and MMP-9 expression. The present study revealed the presence of negative correlation between proliferative activity of cells of the anterior corneal epithelium and a degree of inflammatory infiltration of the corneal stroma; this is evidenced by studying the expression of corneal repair markers Ki-67, CD 68 and MMP-9. When inflammation is present, proliferative activity of cells of the anterior corneal epithelium decreases; therefore, the rate of epithelization and following lamellar cell differentiation is reduced. It should be noted, that expression of the studied immune histochemical markers in the cornea was similar in both groups. Essential differences were noted only at the sites of the sutures. Inflammatory process and the reduction in epithelization rate was more common for securing AM to the corneal surface with sutures, in experimental animals, in which lamellar AMT was used.
References 1.Hao Y, Ma DH, Hwang DG, Kim WS, Zhang F. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea. 2000;19:348-52. 2.Kjaergaard N, Hein M, Hyttel L et al. Antibacterial properties of human amnion and chorion in vitro. Eur J Obstet Gynecol Reprod Biol. 2001;94:224–9. 3.Talmi WP, Sigler L, Inge E, Finkelstein Y, Zohar Y. Antibacterial properties of human amniotic membranes. Placenta. 1991;12:285-8. 4.Tseng SC, Li DQ, Ma X. Suppression of transforming growth factor-beta isoforms, TGF-beta receptor type II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix. J. Cell Physiol. 1999;179:325-35. 5.Heiligenhaus A, Li H, Yang Y, Wasmuth S, Bauer D, Steuhl KP. Amniotic membrane transplantation improves experimental herpetic keratitis. Modulation of matrix metalloproteinase-9. Ophthalmologe. 2004;101(1):59-65. In German. 6.Bauer D, Wasmuth S, Hennig M, Baehler H, Steuhl KP, Heiligenhaus A. Amniotic membrane transplantation induces apoptosis in T lymphocytes in murine corneas with experimental herpetic stromal keratitis. Invest. Ophthalmol. Vis. Sci. 2009;50(7):3188-98. 7.Barequet Irina S, Habot-Wilner Z, Keller N, Smollan G, Ziv H, Belkin M, Rosner M. Effect of Amniotic Membrane Transplantation on the Healing of Bacterial Keratitis. IOVS. Jan 2008;49:163-7. 8.Gicquel JJ, Bejjani RA, Ellies P, Mercie M, Dighiero P. Amniotic membrane transplantation in severe bacterial keratitis. Cornea. 2007;26:27-33. 9.Kim JS, Hahn TW, Park WC. Amniotic membrane transplantation in infectious corneal ulcer. Cornea. 2001;20:720–6. 10.Bauer D, Hennig M, Wasmuth S, Baehler H, Busch M, Steuhl KP, Thanos S, Heiligenhaus A. Amniotic membrane induces peroxisome proliferator-activated receptor-? positive alternatively activated macrophages. Invest Ophthalmol Vis Sci. 2012;53(2):799-810. 11.Bromley M, Rew D, Becciolini A et al. A comparison of proliferation markers (BrdUrd, Ki-67. PCNA) determined at each cell position in the crypts of normal human colonic mucosa. Eur. J. Histochem. 1996;40:89-100. 12.Smorodinova N, Kaltner H, Jirsov? K, Hrdli?kov?-Cela E, Andr? S, Ku?era T, Smetana K Jr., Gabius HJ. Regulatory Impact of Amniotic Membrane Transplantation on Presence of Adhesion/Growth-Regulatory Galectins-1 and -7 in Corneal Explants from Acanthamoeba Keratitis Patients: Clinical Note. Curr Eye Res. 2015;4:1-7. 13.Bruno S, Darynkiewich Z. Cell cycle dependent expression and stability of the nuclear protein by Ki-67 antibody in HL-60 cells. Cell. Prolif. 1992;25:31-40. 14.Holt PR, Moss SF, Kapetanakis AM. Is Ki-67 a better proliferative marker in the colon then proliferating cell nuclear antigen? Cancer. Epidimiol. Biomarkers. Prev. 1997;6:131-5. 15.Dong Z, Zhou X, Wu J, Zhang Z et al. Small incision lenticule extraction (SMILE) and femtosecond laser LASIK: comparison of corneal wound healing and inflammation. Br J Ophthalmol. 2014;98(2):263-9. 16.Fabiani С, Barabino S, Rashid S, Reza Dana M. Corneal Epithelial Proliferation and Thickness in a Mouse Model of Dry Eye. Exp Eye Res. 2009;89(2):1166-1171. 17.Tandon R, Gupta N, Kalaivani M, Sharma N, Titiyal JS, Vajpayee RB. Amniotic membrane transplantation as an adjunct to medical therapy in acute ocular burns. Br J Ophthalmol. 2011;95:199-204. 18.Tseng SC, Di Pascuale MA, Liu DT, Gao YY, Baradaran-Rafii A. Intraoperative mitomycin C and amniotic membrane transplantation for fornix reconstruction in severe cicatricial ocular surface diseases. Ophthalmology. 2005;112:896–903. 19.Kenney MC, Chwa M, Lin B, Huang GH, Ljubimov AV, Brown DJ. Identification of cell types in human diseased corneas. Cornea. 2001;20(3):309-16. 20.Liu T, Zhai H, Xu Y, Dong Y, Sun Y, Zang X, Zhao J. Amniotic membrane traps and induces apoptosis of inflammatory cells in ocular surface chemical burn. Mol Vis. 2012;18 (2):137-46. 21.Ollivier FJ, Gilger BC, Barrie KP et al. Proteinases of the cornea and preocular tear film. Vet Ophthalmol. 2007;199–206. 22.Xiaoyong Y, Mitchell BM, Wilhelmus KR. Expression of Matrix Metalloproteinases during Experimental Candida albicans Keratitis. Invest Ophthalmol Vis Sci. 2009;50(2):737-42. 23.Sereda EV, Drozhzhyna GI, Gaidamaka TB. Экспериментальная модель бактериального кератита средней степени тяжести. Oftalmologiia. Vostochnaia Evropa. 2015;2(25); 41-8. In Russian.
|