J.ophthalmol.(Ukraine).2016;1:27-30.
|
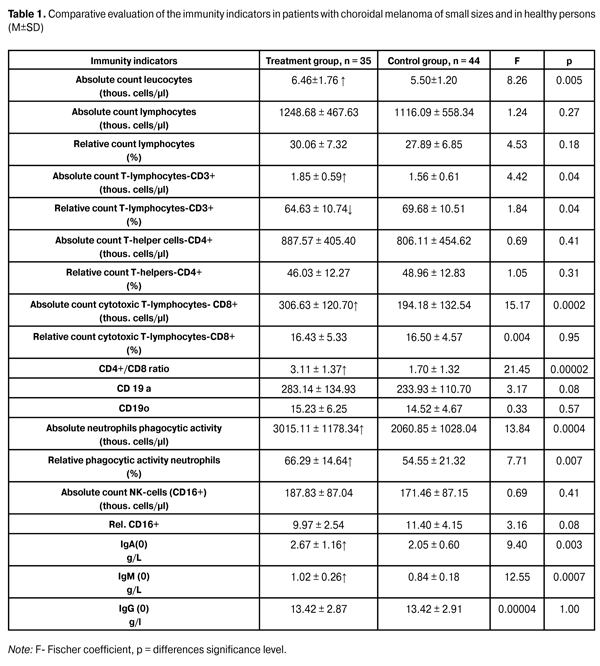
https://doi.org/10.31288/oftalmolzh201612730 Natural antitumor resistance of the organism condition of patients with uveal melanoma of small sizes S.I. Polyakova, Doctor of Medical Sciences L.N. Velichko, PhD in Medical Sciences A.V. Bogdanova, PhD in Biological Sciences I.V. Tsukanova, Junior Research Associate State Institution “The Filatov Institute of Eye Diseases and Tissue Therapy of the National Academy of Medical Sciences of Ukraine” Odessa (Ukraine) E-mail: inna.sister@mail.ru Introduction. The role of immune mechanisms in the tumor process development and progression is currently beyond any doubt. In this regard, the study of the immune system of patients with choroidal melanoma (CM) is very important at the initial stage of the disease specifically, i. e. CM of small sizes (protruding up to 3 mm), that in general can have a positive effect in the implementation of therapeutic pathomorphism in applying various treatment methods. Purpose. To examine the immune system condition in patients with CM of small sizes in comparison with healthy individuals control group. Materials and Methods. An immunological research was implemented in 35 patients with UM of small sizes before the treatment. These were the patients of SI "The Filatov Institute of Eye Diseases and Tissue Therapy of NAMS of Ukraine" (treatment group) and 44 healthy people (control group). The average age of the patients in the treatment group and the control group was (53.9 ± 12.1) and (55.4 ± 11.5) respectively. In the treatment group, there were 26 women (74.3%) and 9 (25.7 %) men. In the control group, there were 26 (59.1%) women and 18 (40.9%) men. In both groups, cellular and humoral immunity components were studied in accordance with generally accepted methods. Results. Most indicators of both cellular and humoral immunity in patients with CM of small sizes were higher than in healthy individuals. So CM-patients at the initial stage of the disease in comparison with healthy people had a statistically significant increase of such indicators, as the absolute cells count (by 17.5%, p = 0.005), T-lymphocytes CD3+ absolute count (18.6%, p = 0.04), cytotoxic cells CD8+ absolute count (57.9%, p = 0.002), immunoregulatory index ratio CD4+/CD8+ (82.9%, p = 0.00002), immunoglobulin A (30.2%, p = 0.003) and M (21.4% p = 0.0007). At the same time, there was a statistically significant minor (2.8%, p = 0.04) decrease of the relative count of T-lymphocytes CD3+. This indicates that at the initial stage of UM progression, patient's immune system is active. An increase of absolute (46.3%, p = 0.0004) and relative (by 21.5%, p = 0.007) phagocytic neutrophils activity also indicates the natural resistance of UM-patients at an early stage of the disease. Conclusions. Uveal melanoma initial stage is accompanied with cellular and humoral specific immunity activation and enhanced antitumor resistance of the organism. Key words: uveal melanoma, choroidal melanoma Introduction Uveal melanoma (UM) is a severe ocular pathology which leads not only to visual function loss and, in some cases, the eye, as the organ of vision itself, but also to the death of the patient as a result of a pleural tumor permeation. Choroidal melanoma (CM) of small sizes, that include CM with height up to 3.0-4.0 mm [2.17], constituting 5-21% of all CM, has a special place among all the UM [14, 16, 17]. CM of small sizes relates to the initial stage of the disease. According to the TNM (2010) classification of human tumors [8], the primary CM - T1 disease stage include CM, protruding into the vitreous up to 6 mm and having the base length of 12 mm, wherein among T1 CM stage there are protruding tumors distinguished up to 3 mm and the base length of 12 mm and protruding tumors from 3 to 6 mm and the base length up to 9 mm. Thus, small tumors under 3 mm can be attributed to choroid melanoma of small sizes. Due to the fact that CM of small sizes is a primary disease stage, a timely adequate treatment provides not only high local efficiency but also a better prognosis for life [1, 9, 15]. The role of immune mechanisms in the tumor process development and progression is currently beyond any doubt. However, the available literature does not contain any studies on subpopulation composition of lymphocytes in patients with CM of small sizes (under 3 mm). Therefore, there is no dependence between the immune system condition of such patients and the therapeutic effect implementation in applying various methods of treatment, in particular, transpupillary thermotherapy. At the same time, the effect of the immune system to the implementation of the therapeutic result has been proven in the process of organ-sparing combination (photocoagulation + biotherapy) treatment of UM [10, 11, 13]. Therefore, it is important in this regard to study the immune system of patients with CM at the initial stage of the disease, which could have a positive effect in the implementation of the therapeutic result. The purpose of this research is to examine the immune system condition in patients with CM of small sizes in comparison with a control group of healthy individuals. Material and Methods An immunological research was implemented in 35 patients with UM of small sizes before the treatment. These were the patients of SI "The Filatov Institute of Eye Diseases and Tissue Therapy of NAMS of Ukraine" (treatment group) and 44 healthy people (control group). The average age of the patients in the treatment group and the control group was (53.9 ± 12.1) and (55.4 ± 11.5) respectively. In the treatment group, there were 26 women (74.3%) and 9 (25.7 %) men. In the control group, there were 26 (59.1%) women and 18 (40.9%) men. Immune system indicators research was conducted in the immunological laboratory of the institute. It was made in accordance with generally accepted methodologies [3, 6, 12], and included the following peripherical blood indicators definition using monoclonal antibodies: 1) absolute leukocytes and lymphocytes count, absolute and relative T-cells CD3+ count; 2) absolute and relative T-helpers CD4+ count; 3) absolute and relative T-suppressors CD8+ count; 4) absolute and relative B-lymphocytes CD19+ count; 5) absolute and relative NK-cells CD16+ count; 6) neutrophils phagocytic activity was determined [3, 6]; 7) A, M and G immunoglobulin count [6]. Statistical processing of the material was made by using ''Statistic 9'' software. Results and Discussion The data on the status of the immunity indicators in patients with CM and the control group of healthy individuals are presented in table 1.
Table 1 shows that most indicators of both cellular and humoral immunity in patients with CM of small sizes are higher than in healthy individuals. So CM-patients at the initial stage of the disease in comparison with healthy people have a statistically significant increase of such indicators, as the absolute cells count (by 17.5%, p = 0.005), T-lymphocytes CD3+ absolute count (18.6%, p = 0.04), cytotoxic cells CD8+ absolute count (57.9%, p = 0.002), immunoregulatory index ratio CD4+/CD8+ (82.9%, p = 0.00002), immunoglobulin A (30.2%, p = 0.003) and M (21.4% p = 0.0007). At the same time, there is a statistically significant minor (2.8%, p = 0.04) decrease of the relative count of T-lymphocytes CD3+. This indicates that at the initial stage of UM progression, patient's immune system is active. An increase of absolute (46.3%, p = 0.0004) and relative (by 21.5%, p = 0.007) phagocytic neutrophils activity also indicates the natural resistance of UM-patients at an early stage of the disease. It is known that malignant tumors, and, in particular, UM, have a significant immunodepressive potential and with their progression, an antitumor resistance of the organism is decreasing. Tumor size increase leads to a decrease in the level of T-lymphocytes (CD3+) and T-lymphocytes-helpers (CD4+). Thus, in patients with total melanoma volume of more than 1000 mm3, T-helpers level is lowered to (31.8 ± 1.7) thous. cells/µl [5,7]. High mortality correlation was also registered in UM with low CD3+ and CD4+ level. At the same time, UM-patients' belonging to various tumor cell types (spindle, epithelioid or mixed) have no significant impact on their immunological reactions [5]. It is reasonable to assume that the immune system activity at the initial stage of the disease in patients with CM is high enough. In this regard, the immune system study under CM of small sizes conditions in the therapeutic effect implementation, especially in connection with the active improvement of diagnostic technologies and respectively, with growing trend to reveal CM at an earlier stage, is rather actual. Conclusions Uveal melanoma initial stage is accompanied with cellular and humoral specific immunity activation and enhanced antitumor resistance of the organism. References 1. Bezrukov AV. [Afterhistory of uveal melanomas]. Tumors and tumor-like ophthalmological diseases: Materials of All-Union conference. Tallinn. 1989:8 - 9. Russian. 2. Bulgakova ES. Treatment of small choroidal melanoma via transpupillary diode-laser thermotherapy method: abstract of thesis for PhD in Medical Sciences: speciality 14.00.08 “Eye Diseases”. Moscow. 2005:124. Russian. 3. Vanichkin AA, Bushueva NN, Degtyarenko TV. et al. [Accelerated primary human immunity status test: methodological recommendations]. Odessa. 1990:23. Russian. 4. Grigoryan SS, Ershov FI. [Clinical efficiency of interferon inducer]. Modern aspects of interferons use. Moscow. 1990:24. Russian. 5. Gusev GA. Use of immunomodulators in complex treatment of malignant tumors of visual organ: abstract of thesis for PhD in Medical Sciences. Moscow. 1991:25. Russian. 6. Degtyarenko ТV. Accelerated primary human immunity status test: methodological recommendations. Odessa. 1990:22. Russian. 7. Ziangirova GG, Likhvantseva VR. [Tumors of eye vascular tract]. Moscow: Poslednee slovo Publ. 2003:456. Russian. 8. [Classification of choroid melanomas]. Oftalmol Zh. 2010;6:20-31. Russian. 9. Libman ES, Brovkina AF, Bezrukov AV. [Afterhistory of uveal melanomas. Comparative assessment of enucleation and organ-sparing treatment modes]. Oftalmol Zh. 1989;6:336 -338. Russian. 10. Maletsky АP. [Characteristics of the immune status of patients with uveal melanomas during organ-sparing treatment]. In: Maletsky AP, Vit VV, Vanichkin AA. Oftalmol Zh. 1989;6:341-345. Russian. 11. Maletsky AP, Terentyeva LS, Degtyarenko TV, Spirko VK. [Prognostic value of clinical and immunological indicators during organ-sparing treatment in patients with choroidal melanomas]. Oftalmol Zh. 2000;4:30-34. Russian. 12. Nazarenko GI, Kishkun AA. [Clinical assessment of the laboratory reports]. Moscow: “Meditsina” Publ. 2000;338:348. Russian. 13. Velichko LN, Maletsky AP, Vit VV, Terentyeva LS. [Status of the system of antitumour autarcesis in patients with uveal melanomas during organ-sparing treatment]. Oftalmol Zh. 1998;2:131-138. Russian. 14. Augsburger JJ, Schroeder RP, Territo C et al. Clinical parameters predictive of enlargement of melanocyte choroidal lesions. Br. J. Ophthalmol. 1989;73:911-917. 15. Limbourg I, Legrain S, De Potter P. Transpupillary thermotherapy for treatment of choroidal melanomas. Bull. Soc. Beige. Ophthalmol. 2002;285:55-64. 16. Zehetmayer M, Kitz K, Menapace R et al. Local tumor control and morbidity after one to three fractions of stereotactic external beam irradiation for uveal melanoma. In: Radiother. Oncol. 2002;55(2):135-144. 17. Shields CL, Shields JA. Transpupillary thermotherapy for choroidal melanoma. Curr. Opin. Ophthalmol. 1999;10(3):197-203. 18. Shields CL, Shields JA. Clinical features of small choroidal melanoma. Curr. Opin. Ophthalmol. 2002;13(3):135-141.
|