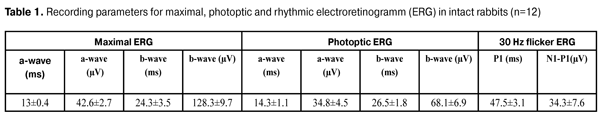
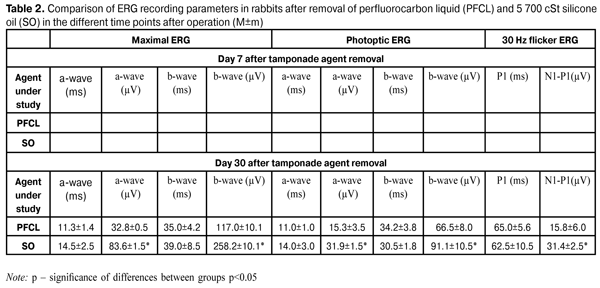
J.ophthalmol.(Ukraine).2016;1:58-64.
|
https://doi.org/10.31288/oftalmolzh201615864 Effect of 30 day tamponade using perfluorocarbon liquid on the bioelectric functional activity and structure of the rabbit's retina Zhmuryk D.V., Cand. of Sc. (Med.) Kyiv Clinical Ophthalmology Hospital Eye Microsurgery Center Kyiv, Ukraine E-mail: visus@ukr.net Introduction. The use of perfluorocarbon liquid (PFCL) for short-term tamponade can broaden retinal detachment surgery indications and improve outcomes. However, there is no common opinion regarding the effect of PFCL on the functional state of the retina. It is relevant to compare the effect of PFCL and light silicone oil (SO). Purpose was to study the effect of 30 day PFCL tamponade on bioelectrical functional activity and structure of the retina in rabbits’ eyes and to compare the actions of PFCL, SO (specific gravity<1.0 g/sm3) and saline solution in process using electroretinography (ERG), light optical microscopy (LOM) and electron microscopy (EM) at different time points after tamponade (7, 14, and 30 days). Material and Methods. LOM, EM and ERG were performed in 12 rabbits (24 eyes). All animals underwent closed subtotal vitrectomy followed by 30 day tamponade using PFCL in the right eyes and light SO and saline solution in the left eyes. Bioelectrical functional activity and structure of the retina in rabbits’ eyes were studied in dynamics at time points of 7, 14 and 30 days after tamponade. Results and Discussion. Dysfunction of first-order neurons in peripheral and central retina was determined; the former was expressed in a decrease in amplitude of biological response to flash by 23-100%, respectively, while the state of second-order neurons in the inner retina was normalized due to PFCL action. The comparison of effects of 30 day tamponade using PFCL and SO (specific gravity<1.0 g/sm3) revealed that SO induced more apparent reactivity of photoptic and scotoptic response in the retina (1.4-2.25 times). The retinal structures had similar changes after 30 day tamponade with the tamponading agents used. However, the alterations were reactive, not damaging, and reversible. Conclusion. PFCL can be used as a tamponading agent for short-term tamponade. Key words: retinal structure, functional activity, electroretinography, perfluorocarbon liquid, silicone oil, experiment Introduction The modern stage in the development of surgical treatment for vitreoretinal pathology has been marked by new achievements. Over the last ten years, there have appeared new equipments to perform vitreoretinal surgery, the latest generation of lasers, and optic devices to visualize clearly the ocular fundus during the surgery. It is constantly that physical and chemical features and biological inactivity of perfluorocarbon liquids (PFCL) are improved and more purified silicone oil (SO) and various vitreoretinal tools are developed. All this made it possible to broaden the indications and extent of surgical operation in the pathology of the posterior segment of the eye. Perfluorocarbon liquids are an important tool in the vitreoretinal surgery, the general development of which is dependent on the development of each part. PFCL are characterized by a specific gravity, which is twice as big as that of water, and they have chemical and metabolic inactivity, transparency and low viscosity, characteristics which are of a great value in the vitreoretinal surgery. The first experience of intravitreal PFCL was reported by Haidt et al. in 1982 [1]. However, vitreoretinal surgeons have a dual attitude towards PFCL tamponade of the vitreous. The issue of injuring action of PFCL and the most secure term of tamponade is open. Up-to-date PFCL are high-purity inactive chemical compounds and the process of their recognition by protective system of the organism can be explained by physical and biological interaction with intraocular structures. Physical exposure is realized through the pressure on the substructure because of the high specific gravity and as a result of inactive movement of PFCL within the vitreous cavity. Biological interaction of PFCL with tissues of the eye may be caused by PFCL oxygenation and their dissolution in cellular membrane lipids. We have performed analyze of experimental papers on this issue and found that their findings are different. A part of researchers have reported on the development of irreversible atrophic changes after PFCL tamponade with the duration of 48 hours [2], two weeks [3], and one month [4, 5, 6]. Other investigations have reported on the absence of significant alterations even after 1 month tamponade [7, 8]. However, they are difficult to compare since the conditions of the experiment are different; in particular, different vitrectomy techniques and agents with various specific gravity have been used, and localization of the most injuring action has not always been considered when sampling. Saline solution has been always used as controls. Based on the findings of previous experiments, it would be relevant: •to make experimental conditions most closely resembling real-life conditions (vitrectomy); •to use PFCL with high specific gravity to perform tamponade; •to study alterations in the retina at different time points after tamponade; •to take into account allocation of maximal injuring action; •to compare the effect of PFCL on the retinal structure with a standard tamponade agent, silicone oil. The purpose of the present study was to determine the effect of 30 day PFCL tamponade on the bioelectrical functional activity and structure of the retina in rabbit’s eye in experiment and to compare the action of PFCL, SO (a specific cavity is <1.0 g/cm3) and saline solution in process using electroretinography (ERG), light optical microscopy (LOM) and electron microscopy (EM) at different time points after tamponade completion (7, 14, and 30 days). Materials and Methods All interventions and euthanasia were performed in accordance with The International Guiding Principles for Biomedical Research Involving Animals developed by the Council for International Organizations of Medical Sciences [9]. A total of 12 male Chinchilla rabbits (24 eyes), weighted (3.5±0.5) kg, aged (6.5±0.5) months were included into the experiment. The duration of tamponade was 30 days. Each animal was performed ERG before the experiment as well as at different time points after PFCL tamponade, i.g. at 7 and 30 days. LOM and EM of the retina were performed to each animal after tamponade using PFCL, SO and saline solution. The experimental animals were divided into three groups according to time points studied: group 1 (4 rabbits; ERG, LOM, EM; 7 days), group 2 (4 rabbits; LOM, EM; 14 days), and group 3 (4 rabbits; ERG, LOM, EM; 30 days). In all cases, the second eye (left) was used as controls. In control eyes, tamponade agents were silicone oil (2 rabbits in each group) with viscosity of 5 700 cSt, specific gravity< 1.0 g/cm3, and saline solution (other 2 rabbits in each group). Surgical technique Preoperatively, to anesthetize the experimental animals, thiopental sodium (2mg/kg) was injected intramuscularly and 0.5% proparacaine was administered epibulbarly; mydriasis was achieved with epibulbar 1% atropine sulfate and 2.5% phenylephrine. Before surgery, 0.3% ofloxacin was used epibulbarly. 20G, 23G and 25G closed subtotal vitrectomy was performed using KFE-01 “MEDA-NN” Sonorus operating system (frequency up to 1 200 bpm; aspiration 150 mmHg). The vitrectomy was performed under the control of OPTONO pMi-8 operating microscope and Photon 2 light. 1.5 mL of perfluorodecalin (PFD) was administered in the vitreous cavity of the right eye. The cavity of the left was filled with 1-1.5 mL of SO or saline solution as controls. After vitrectomy, 0.3% ofloxacin ointment was put into the conjunctival cavity. The tamponade duration was 30 days. Tamponade was completed with preoperative operations described above. Removal of PFD was performed using KFE-01 “MEDA-NN” Sonorus operating system (frequency up to 1 200 bpm) under the control of OPTONO pMi-8 operating microscope and Photon 2 light. Silicone oil was removed actively under the operating microscope control. On the eyes with saline solution tamponade, the latter was replaced by new saline solution to keep the same conditions of operations in animals with PFD and SO. ERG procedure Preoperatively, 0.5% proparacaine was administered epibulbarly; mydriasis was achieved with epibulbar 1% atropine sulfate and 2.5% phenylephrine. A full field (Ganzfeld) ERG examination was performed using electrophysiological test unit Retiscan according to ISCEV Standard ERG protocols, which included scotoptic response, photoptic response, and photoptic response to 30 Hz flicker. The contact lens electrode was placed on the cornea; the reference electrode was placed on the forehead skin at the medium line; and the ground electrode was placed to the ear skin. The full field ERG indicates the summarized electric response of the retina, stimulated by light flashes at the surface of the Ganzfeld bowl (Ganzfeld stimulus). Ganzfeld ERG indices, including scotoptic response (Maximal ERG), photoptic response (Photoptic ERG) and photoptic response to 30 Hz flicker (Flicker ERG), were determined at time points of 7 and 30 days after tamponade. LOM and EM procedure The ultrastructure of the retina was examined using light optic and electron microscopy at time points of 7, 14, and 30 days after tamponade completion. To perform LOM, at the end of the experiment, the retinas obtained from enucleated eyes were fixed in the 10% neutral formalin and embedded in paraffin. Sections were stained with hematoxylin and eosin. Light optical microscopy was performed using a microscope Jenamed 2. To perform EM, sections of the retina (lower segments in PFCL tamponade and upper segments in SO (viscosity 5 700 cSt)) were fixed in 2.5% glutaraldehyde in phosphate buffer (рН – 7.4) with following postfixation with 1% osmic acid in the same pH buffer. Afterwards, the samples were dehydrated in graded alcohol solutions. Araldite/Epon mixture was used to infiltrate and embed the samples. Then, ultrathin segments were contrasted according to Reynolds [10]. Electron microscopy was performed using an electron microscope PEM-100 – 01. Results and Discussion Response of the retina to 30 day PFCL tamponade Ganzfeld ERG parameters, including scotoptic combined response (Maximal ERG), photoptic response (Photoptic ERG) and photoptic response to 30 Hz flicker (Flicker ERG) were recorded at time points of 7 and 30 days after the removal of PFCL and SO tamponading agents. At Day 7 after PCFL removal, Maximal ERG revealed that implicite time and a-wave amplitude was not significantly changed as compared to controls, indicating a stabilized response to the light stimulus by photoreceptor layer of the peripheral retina. The response to the stimuli by the inner layers of the retina was characterized by nearly doubled b-wave implicit time (48.8±5.8 ms (р=0.001)) and an increase of b-wave amplitude to 215.5±7.5 (µV), (р=0.001) (Tables 1, 2).
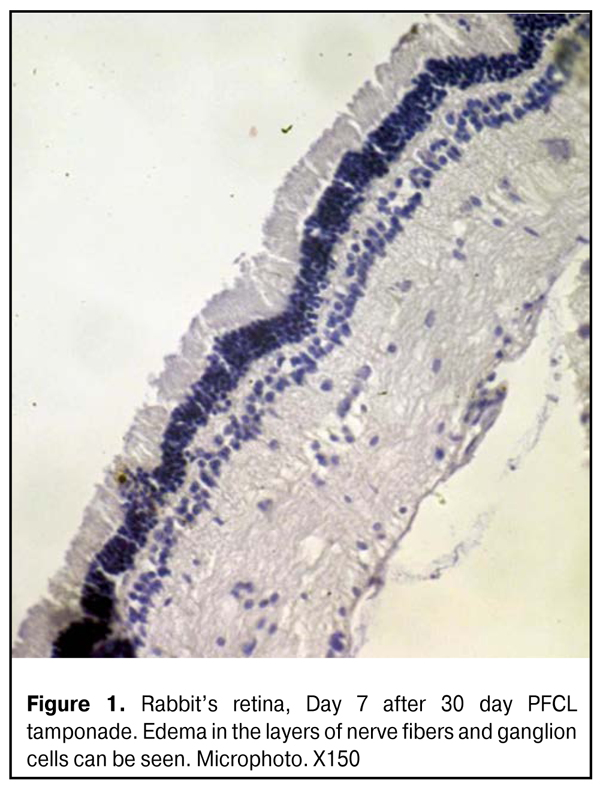
Parameters of photoreceptor layer in the central retina didn’t differ significantly, according to the data of Photoptic ERG. Furthermore, inner retina activity was not significantly changed (b-wave parameters). Thus, b-wave amplitude was not increased significantly as compared to initial one and was equal to 80.5±7.5 (µV); however, implicit time increased to 40.2±2.2 (ms) (р=0.001). Flicker ERG revealed an increase of photoptic response N1-P1 wave amplitude to 47.0±3.0 (µV) (р=0.07) and increased implicit time (63.2±2.1 ms (р=0.01)) (Tables 1, 2). Hence, a week after PFCL removal, a reaction of response activation to light stimulus was revealed in the middle retinal layers; that was manifested in increased ERG amplitude (supernormal ERG) though accompanied by decreased potentials. Thus, there was detected excessive irritability of the retina, i.e. changes in the physiological parameters, increasing their offset without prior external exposure. LOM didn’t reveal any significant changes in the retinal structure, despite edema in the nerve fiber and ganglion cell layers, at all time points (Fig.1).
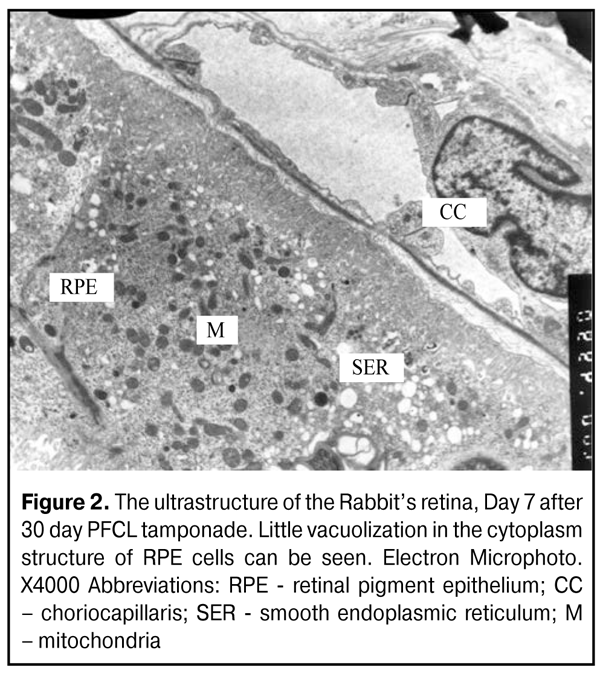
At Days 7-14, the ultrastructure of the retinal pigment epithelium (RPE) didn’t differ from the normal one, despite single cells, in which cytoplasm structure vacuolization was observed. No alterations were seen in photoreceptor cells (PC) at this time point. Neurons and nerve structures of the inner retinal layers didn’t differ as well. In cytoplasm of M?ller cell process (MCP) in the inner limiting membrane (ILM), osmiophilic granules were noted, that made the structure of MCP different from the normal one (Fig.2).
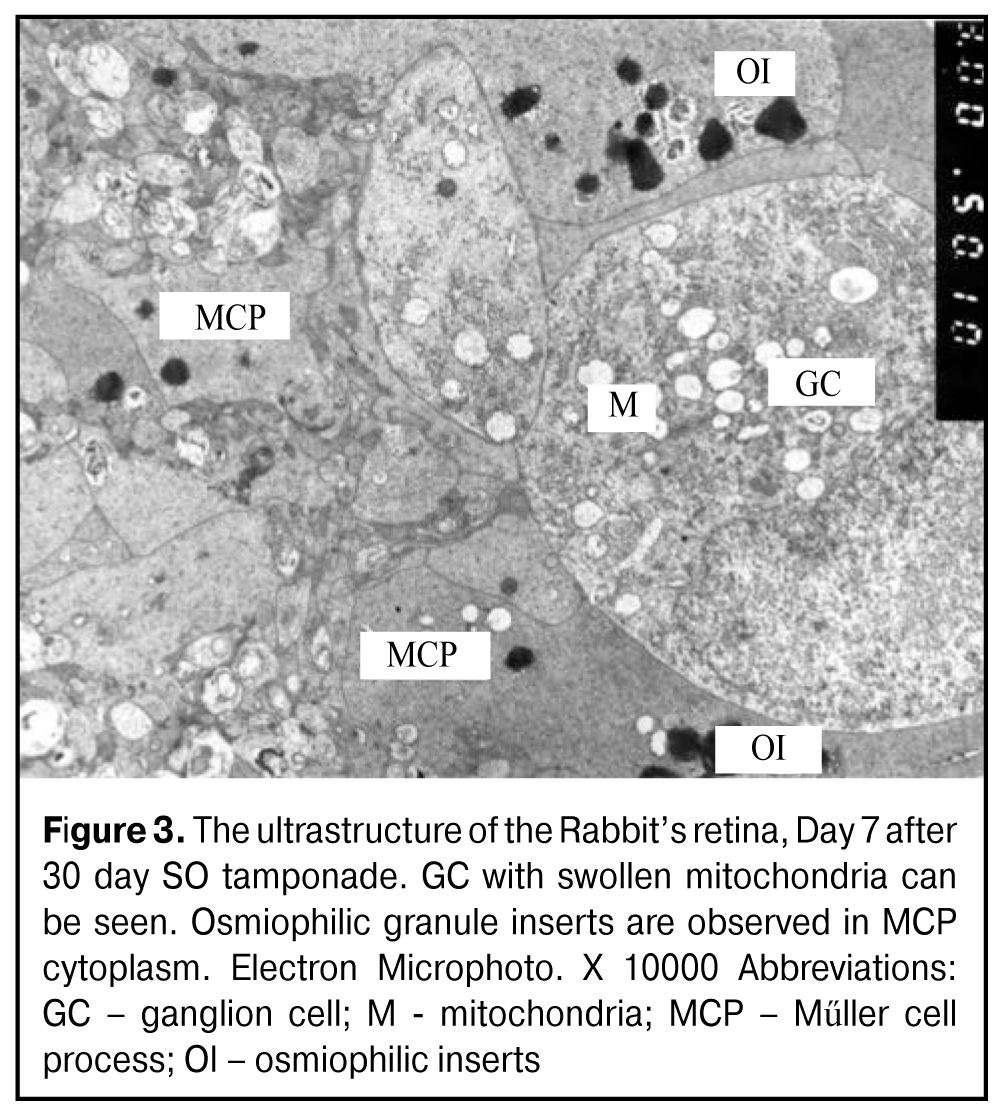
At Day 30, the retinal structure studied didn’t differ from the norm. At Day 30 after PFCL removal, Maximal ERG a-wave time decreased from 14.5±1.5 (ms) to 11.3±1.4 (ms) (р=0.01) as compared to that at Day 7. A-wave amplitude also decreased to 32.8±0.5 (µV); that was lower than the norm (р=0.01) and confirmed the dysfunction of photoreceptor layer in the peripheral retina at Day 30 of postop follow-up. Inner retina activity on b-wave amplitude decreased by 45.5% (р=0.001) for 2 weeks, and was almost resolved (117.1±10.1 (µV) (р=0.01); this characterizes the safety of second-order neurons. Photoptic retinal system at Day 30 was characterized by reduced action potential duration in the photoreceptor layer by 29% (p=0.01) and by significantly reduced amplitude (2.6 times) as compared to the previous time point of 7 days; thus, it was equal to 15.3±3.5(µV) and twice as small as the controls. This indicates the dysfunction of first-order neurons in the central retina. Inner retina activity decreased by 17.4% (p=0.01) achieving the norm values; this indicates the excessive irritability reversal as a response to PFCL action by second-order neurons. Hence, the remote follow up of the functional state of the retina after PFCL removal after 30 day tamponade revealed moderate dysfunction in first-order neurons, i.g. response amplitude was decreased two times in the central part and by 23% in the periphery; while the functional state of second-order neurons was normalized. At Day 30 after tamponade completion, the retinal structure studied didn’t differ from the norm. Retina response to 30 day SO tamponade Comparative analyzes of bioelectrical retina activity after the removal of tamponading agents PFCL and SO (specific gravity<1.0 g/sm3) demonstrated that Maximal ERG a-wave amplitude was two times increased in SO group at Day 7 (р=0.001) (Table 2), and twice as much as the norm (р=0.001). Biological activity of the inner peripheral retina was characterized by increased reactivity on b-wave amplitude by 26.9% compared to that after PFCL removal (Table 2). In SO group as in PFCL group, inner retina activity in the central part increased by 54% (р=0.001) as compared to the norm (Tables 1, 2). At Day 7, the structure of the most RPE cells had no apparent changes; however, part of them had various caliber vesicles and bigger electron-transparent excavations in the cytoplasm, i.g. signs of insignificant hydroptic alterations in cytoplasm structure were observed. No changes were noted in photoreceptor cells and in neurons of other retinal layers. Round inserts were observed in MCP cytoplasm near ILM; the former consisted of osmiophilic granule closely compacted and surrounded by electron-transparent rim. These inserts often were bigger than those in PFCL tamponade (Fig. 3).
At Day 14 after SO tamponade, there was detected severe retinal detachment appeared to be associated with manipulations during the experiment. They also caused hydroptic alterations and breaks in RPE cells as well as a rupture of RPE from NLFC. Photoreceptor cells and neurons of the retina were not changed, in general. At Day 30 after tamponade, the retinal structure studied didn’t differ from the norm. At Day 30 after SO removal, photoreceptor layer reactivity in the peripheral retima insignificantly decreased by 15.5%, increasing the norm 1.96 times (p=0.001). Maximal ERG showed high inner peripheral retina activity which was twice as much as the norm (р=0.001). This group was characterized by the positive dynamics expressing in normalization of central retina photoreceptor layer bioelectrical activity; however, inner retina activity was still increased as compared to the norm (p=0.001) and was equal to 91.5±10.5 (µV). In group with SO tamponade, reactivity of photoreceptor layers in the central retina and of inner peripheral retinal layers were increased 2.1-2.5 times and 1.37-2.2 times, respectively, as compared to PFCL tamponade group. At Day 30 after tamponade, the retinal structure studied didn’t differ from the norm. Retina response to 30 day SS tamponade LOM revealed no significant retinal changes (Fig. 4)
After 30 day SS tamponade, all retinal elements studied were visually normal at time points of 7 and 30 days. Discussion At Day 30 after 30 day PFCL tamponade, subnormal ERG which is characterized by a decrease of a-wave amplitude was noted; this can be evidence of the beginning of dystrophic processes in the photoreceptor layer. After SO tamponade at the same time point, subnormal ERG on b-wave amplitude in photoreceptor and inner layers in the central and peripheral retina was still present; this fact indicates the insufficient recovery of biological activity in the retina, i.g. its high irritability. According to the data of our previous studies, where we have analyzed functional activity of the retina in tamponade with various durations, supernormal ERG has been observed after 7 day tamponade using PFCL and light SO. We suppose this is the reaction to surgical intervention and drug injection. And retinal activity has been almost within the norm one month later [4]. In 14 day PFCL and heavy SO tamponades, supernormal ERG has been also present; however, the indices have not returned to the norm within 1 month after tamponade [5]. At Day 30 after 30 day tamponade, dysfunction (initial dystrophy) in peripheral retina photoreceptor layers has been observed when using PFCL and retina hyperactivity (dysfunction) has occurred when using SO. It is impossible to compare our findings with those of other authors since the experimental conditions are different, agents used have different specific gravity, and due to the presence of a tamponading agent in the eye. So, Zeana D. et al. have reported on some decrease of ERG amplitude after 14 week PFCL tamponade [11]. Flores-Aguilar M. Et al have also determined ERG amplitude reduction after 3 month tamponade; and this has been explained by shielding properties of PFCL. These properties have been also described for SO [1]. Mackiewicz J. Et al have revealed the absence of ERG parameter changes after 3 months tamponade [12]. Since experimental papers mentioned have been performed without tamponade completion, their data cannot be compared with our data. EM revealed mild reactive hydroptic changes in cytoplasm of some RPE cells at time point of 7 days after 30 day PFCL and SO (specific gravity <1.0 g/sm3) tamponade. At time points of 14 and 30 days, the structure of retinal elements studied was nearly within the norm. However, it should be pointed that round formations containing osmiophilic granules were noted in the cytoprasm of MCPs near ILM at time points of 7 and 14 days. These can be secondary lysosomes utilizing foreign material intracellular. It is difficult to say whether it is related to the utilization of tamponading agents. The structure of the retina is nearly unchanged when saline solution is used. Conclusion The features of the PFCL effect on the functional activity in the rabbit’s retina were determined in dynamics within one month after 30 day PFCL tamponade. Dysfunction of first-order neurons in photoreceptor layer in peripheral and central retina was determined; the former was expressed in a decrease in amplitude of biological response to flash by 23-100%, respectively, while the state of second-order neurons in the inner retina was normalized due to PFCL action. The comparative analyze of effect of 30 day tamponade using PFCL and SO (specific gravity<1.0 g/sm3) revealed that SO induced more apparent response reactivity in the central and peripheral retina (1.4-2.25 times).
Since 30 day PFCL tamponade does not influence significantly on the retinal ultrstructure and are comparable to a standard wide-used tamponading agent, silicon oil (specific gravity<1.0 g/sm3), PFCL can be used as a tamponading agent for short-term tamponade. References 1. Flores-Aguilar M., Munguia D., Loeb E., Crapotta J.A.,Vuong C., ShakibaS., Bergeron-Lynn G., Wiley C.A., Weers J., Freeman W.R. Intraocular tolerance of perfluorooctylbromide (perflubron).Retina.1995; 1:3-13. 2. Chang S. Sparrow J.R., Iwamoto T, Gershbein A., Ross R., Ortiz R. Experimental studies of tolerance to intravitreal perfluoro-n-octane liquid. Retina.1991;4: 367-374. 3. Shkvorchenko DO, Levina LV. [On the tactic of surgical treatment of proliferative diabetic retinopathy, complicated by anterior proliferative vitreoretinopathy]. Oftalmokhirurgija. 2006: (1): 29-32. Russian. 4. Zhmuryk DV, Hramenko N, Slobodjanik SB, Milienko MV. [Experimental study of the influence of short-term tamponade with perfluorine organic compounds on the bioelectric functional activity of the retina of the rabbit eye].Oftalmol.Zh. 2014; (1): 86-92. Russian. 5. Zhmuryk DV, Milienko MV. [Experimental study of the influence the two-week tamponade with perfluorine organic compounds on the bioelectric functional activity of the retina of the rabbit eye]. Oftalmol.Zh. 2014; (4): 80-87. Russian. 6. Stolba U., KreplerK., Velikay-Parel M., Binder S. The effect of specific gravity of perfluorocarbonliquid on the retina after experimental vitreous substitution. Graefes Arch. Clin. Exp. Ophthalmol. 2004; 11: 931-6. 7. Brett Drury, Robert D Bourke. Short-term intraocular tamponade with perfluorocarbon heavy liquid. Br J Ophthalmol 2011;95(5):694-8. 8. Haidt S.J., Clark L.C., Ginsberg G. Liquid perfluorocarbon replacement of the eye. Invest. Ophthalmol.Vis. Sci. 1982; 22: 233. 9. Norman H.J. [The International Guiding Principles for Biomedical Research Involving Animals]. Chronicles WHO. 1985; Т.39, 3: 3-9. 10. Sirimaharaj M., Balachandran C., Chan W.C., Hunyor A.P., Chang A.A., Gregory-Roberts J., Huny A.B., Playfair T.J. Vitrectomy with short termpost operative tamponade using perfluorocarbon liquid for giant retinal tears. Br. J.Ophthalmol. 2005; 9: 1176-1179. 11. Zeana D., Becker J., Kuckelkorn R., Kirchhof B. Perfluorohexyloctane as a long-term vitreous tamponade in the experimental animal. Experimental perfluorohexyloctane substitution. Int. Ophthalmol. 1999; 23: 17-24. 12. Mackiewicz J., Maaijwee K., Luke C. Effect of gravity in long-term vitreous tamponade: in vivo investigation using perfluorocarbon liquids and semi-fluorinated alkanes. Graefes Arch. Clin. Exp. Ophthalmol. 2007; 245: 665–675.
|