J.ophthalmol.(Ukraine).2015;6:42-45.
|
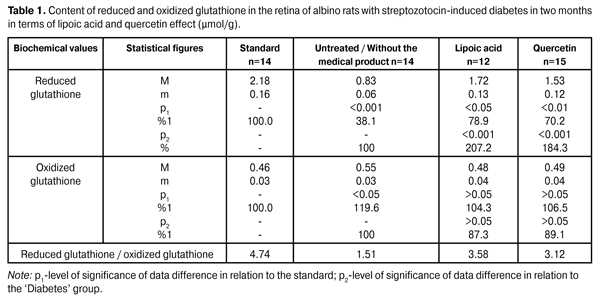
https://doi.org/10.31288/oftalmolzh201564245 Quercetin and lipoate effect on the glutathione system in retina in terms of diabetes simulation O.A. Moroz, PhD in Medical Sciences А. Novak Transcarpathian Regional Clinical Hospital Ophthalmology Department (Uzhgorod, Ukraine) E-mail: moroz.oleg@gmail.com Keywords: streptozotocin-induced diabetes, retina, glutathione, quercetin, lipoic acid, experimentIntroduction. The relevance of the work lies in the research of the quercetin and lipoate effect in terms of experimental diabetes treatment. Objective. To study the quercetin and lipoate effect on the glutathione system in retina in terms of diabetes progress process (in two and six months). Materials and methods. The research was conducted using albino rats. Experimental animals were divided into four groups: the first one – control group (14 rats); the second one – experimental group (14 rats), untreated animals with developing diabetes; the third one – experimental group (12 rats), animals with developing diabetes and lipoic acid administration; the fourth one – experimental group (15 rats), animals with developing diabetes and quercetin administration. Reduced and oxidized glutathione content was determined in retinal homogenates. Results. Lipoic acid and quercetin use enhanced the reduced glutathione level by 107.2 and 84.3% in 2 months in terms of research, and by 134.8% and 113.0 in 6 months after the diabetes progress compared to the group of untreated animals. Conclusion. In terms of streptozotocin-induced diabetes progress the decrease of coenzyme (reduced) glutathione form by 61.9% (in 2 months) and by 68.3% (in 6 months) was determined. Lipoic acid and quercetin medical products administration had a normalizing effect on the retinal metabolism, which is manifested in the reduced glutathione levels increase by 107.2 and 84.3% (in 2 months) and by 134.8 and 113.0% (in 6 months). The lipoic acid application creates a more distinct normalizing effect on the glutathione level. Introduction Due to the fact that the number of diabetic patients increases, diabetic retinopathy has become one of the leading causes of visual impairment and blindness [1]. The absence of clear understanding regarding the diabetes basic complications mechanisms inhibits the development of effective treatment and prevention methods of diabetic retinopathy [3, 12, 16]. In this regard, the broad front of research aimed at the understanding of diabetic retinopathy molecular mechanisms key is deployed at the moment, as a meaningful research is possible only in this field regarding the effective treatment and prevention methods of this disease [7, 17, 20, 22]. It must be noted that until recently the primary focus of diabetic retinopathy pathogenesis was aimed at the glycosylation end products, whereas previously metabolic disorders that lead to hydroxyaldehydes accumulation and antioxidant system capacity decrease were considered as additional factors in the pathogenesis of this disease. At present a considerable progress was made regarding the research of the diabetes molecular basis and its accompanying complications, as well as the role of this process’ early products was disclosed. It was established that a high level of glucose causes metabolic disorders circuit both inside cells and in the extracellular space [5, 13, 19]. Not only an increased glucose level is considered to be the launching metabolic disorder that results in lesions of vascular nerve tissues and other body tissues, but also an increased level of a number of carbohydrate-phosphorus and lipid metabolism metabolites, such as methylglyoxal, acetoacetate, diacylglycerol, deoxyglucose and others. Increasing concentrations of these metabolites not only adversely affect the metabolism state, but also cause cell metabolism and function dysregulation. Then a sharp activation of free radical oxidation processes occurs, at this the antiradical system of the body is not able to completely neutralize toxic radicals, resulting in their excessive amount causing damage to the membrane structure, proteins, lipids, nucleic acids and etc. [2, 4, 14]. Glutathione oxidation-reduction system is the most important element of detoxicative body system and eye tissues, which in particular neutralizes the above mentioned toxic metabolites and quenches free radicals [18]. This tripeptide’s protective role lies not only in detoxification and antioxidant function, but is also is of importance due to the glutathione value in the inflammatory and immune processes regulation and its antiviral effect [21]. With the consideration of detoxicative and membrane stabilizing properties of glutathione, its level plays an important role regarding the intraocular structures protective and adaptive mechanisms in terms of pathological conditions [18]. Meanwhile, the data obtained regarding this system during the diabetes progress are contradictory in a variety of cases and are extremely insufficient in terms of diabetic retinopathy. In particular, in the course of experimental studies it was established that during the initial progress period of the streptozotocin-induced diabetes the glutathione content sharp decrease is marked in 28 days in the experimental animals’ retina, primarily due to the concentration decrease of its reduced form. Noted disorders of glutathione system in the animals’ eyes retina in terms of diabetes simulation can certainly be seen as an important link of the diabetic retinopathy pathogenesis [9, 11]. It must be noted that an important role is played by the glutathione-peroxidase reaction stimulation that neutralizes lipid hydroperoxides, the level of which is known to significantly increase in terms of diabetes [10]. In this regard, it appears relevant to examine the level of glutathione oxidized and reduced forms in the eye retina in a more remote period of time (two and six months) of the experimental diabetes progress when clear signs of diabetic retinopathy are already observed. Work objective: To study the quercetin and lipoate effect on the glutathione system in retina in the process of diabetes progress (in two and six months). Materials and methods Research was conducted using albino Wistar rats with 180 – 210 g weight. Experimental animals were divided into four groups: the first one – control group (14 rats); the second one – experimental group (14 rats), untreated animals with developing diabetes; the third one – experimental group (12 rats), animals with developing diabetes and lipoic acid administration; the fourth one – experimental group (15 rats), animals with developing diabetes and quercetin administration. Quercetin and lipoic acid were orally administered to animals with developing diabetes during the whole experimental period. The work with the animals was carried out in accordance with international guidelines for biomedical research involving animals proposed at the Council of International Organizations of Medical Sciences (2012). The diabetes was induced by means of streptozotocin injection (55 mg per 1 kg of body weight, intraperitoneal). In two months of diabetes progress a part of animals (separate groups) that participated in the experiment under different conditions, as well as normal rats (control group) were decapitated with the preliminary use of the thiopental sodium preanesthetic (50 mg of the medical product per kg of weight). The eyes were enucleated on the ice at 0–5?С temperature. In six months of diabetes progress the remaining part of animals, which still was used in the course of experiment under different conditions, was also eliminated in accordance with the relevant regulations. The animals’ removed retina was immediately provided for the research. Reduced and oxidized glutathione content determination in the retina homogenates was conducted. The principle of the glutathione determining method is based on the reaction between glutathione and methylglyoxal under the glyoxylate enzyme influence, resulting in the formation of a glutathione S-lactam conjugate, which has a maximum absorption at a wave length of 240 nm. The principle of the oxidized glutathione determining method consists in the enzymatic reduction of oxidized glutathione with glutathione reductase using the reduced form of nicotinamide adenine dinucleotide phosphate (NADPH), the decline of which is recorded spectrophotometrically at 340 nm wave length. The mean variation coefficient value for the specified range of the reduced form amounts to 4.0% and 5.0% as for the oxidized form. SF-26 spectrophotometer was used to conduct the measurements [8,15]. The obtained data were statistically processed using SPSS 11.0 package [6]. Results and their discussion The data on the reduced and oxidized glutathione content in retina of albino rats with streptozotocin-induced diabetes in 2 months after being exposed to lipoic acid and quercetin are outlined in Table 1.
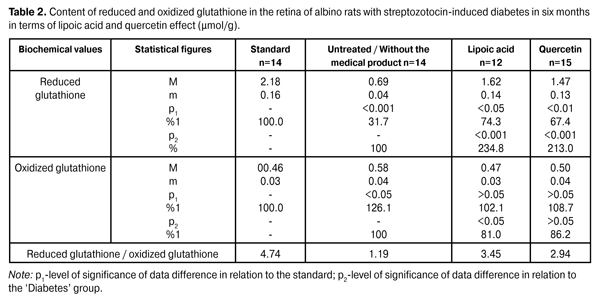
As is evident from reported data the reduced glutathione content in the untreated diabetic animals’ retina has reduced to 61.9% with respect to the standard of (2.18 ± 0,16) µmol/g. In terms of lipoic acid application in two months after the diabetes progress the reduced glutathione content in the retina has increased up to (1.72 ± 0.13) µmol/g; that is it increased by 107.2% (p <0.001) compared to the ‘untreated’ group (0.83 ± 0.06) µmol/g. In terms of quercetin effect the reduced glutathione content has increased by 84.3% (1.53±0.12) µmol/g with respect to the group animals that did not receive the medical product (р<0.001). In two months of the diabetes progress in terms of lipoic acid application the reduction of oxidized glutathione content down to (0.48 ± 0.04) µmol/g is indicated in the retina, which amounted to 87.3% compared to the ‘untreated’ group (0.55 ± 0.03) µmol/g. In terms of quercetin effect the oxidized glutathione content has decreased with respect to the group of untreated animals by 10,9%, i.e. (0.49 ± 0.04) µmol/g. The oxidized glutathione content in the group of untreated animals has increased by 19,6% compared to the standard of (0.46 ± 0.03) µmol/g. The data on the reduced and oxidized glutathione content in albino rats’ retina with streptozotocin-induced diabetes in 6 months after being exposed to lipoic acid and quercetin are outlined in Table 2.
In six months of the diabetes progress in terms of lipoic acid application the oxidized glutathione content in the retina has increased up to (1.62±0.14) µmol/g, i.e. it has increased by 134.8% (p <0.001) compared to an ‘untreated’ group (0.69 ± 0.04) µmol/g. In terms of quercetin effect the reduced glutathione content has increased by 113.0%, which amounted to (1.47±0.13) µmol/g with respect to the group animals that did not receive the medical product (р<0.001). The reduced glutathione content in the diabetic untreated animals group has reduced to 68.3% with respect to the standard of (2.18 ± 0.16) µmol/g. The oxidized glutathione content in the retina in terms of experimental diabetes progress and lipoic acid application was reduced to (0.47 ± 0.03) µmol/g, i.e. it amounted to 81.0% with respect to the untreated group of diabetic animals (0.58 ± 0.04) µmol/g (p<0.05). In the group of diabetic animals in terms of quercetin application the content of oxidized glutathione has decreased by 13.8%, i.e. (0.50 ± 0.04) µmol/g, compared to the ‘untreated’ group. The oxidized glutathione content in terms of diabetes without the medical product application has increased by 26.1% compared to the standard of (0.46 ± 0.03) µmol/g. Analysis of the research being conducted on our part shows a sharp decline in the glutathione system reduction potential, primarily due to a sharp reduction of the coenzyme reduced form level. Thus, the concentration of reduced glutathione form in two months of the diabetes progress has reduced by 61.9%, and in six months – by 68.3% without the medical product application. The obtained data provide grounds to believe that the main metabolic targets, on which it is necessary to focus while developing the diabetic retinal lesions prevention and treatment methods, are the following: the antioxidant system potential reduction, in particular, the reduced glutathione concentration reduction that contributes to the lipid peroxidation products accumulation in the retina and the retinal pigment epithelium intracellular membranes disintegration (lysosomes labilization). In our opinion, the glutathione levels normalization should contribute to the methylglyoxal neutralization in glyoxalase reaction with the use of glutathione. Also, the glutathione system potential normalization activates the free radical compounds quenching processes due to the reduced glutathione form. On the basis of the above, we have conducted research in order to identify the possibility of described metabolic processes correction by means of lipoic acid and quercetin medical products. Lipoic acid and quercetin application allowed to increase the reduced glutathione level by 107.2 and 84.3% in two months of the research and by 134.8 and 113.0% in six months of the diabetes progress as compared to the untreated animal group. It is noteworthy that lipoic acid has a more distinct normalizing effect on the retinal reduced glutathione concentration. Conclusions 1.In terms of streptozotocin-induced diabetes progress the decrease of coenzyme (reduced) glutathione form by 61.9% (in 2 months) and by 68.3% (in 6 months) was determined. 2.Lipoic acid and quercetin medical products administration had a normalizing effect on the retinal metabolism, which is manifested in the reduced glutathione levels increasing by 107.2 and 84.3% (in 2 months) and by 134.8 and 113.0% (in 6 months). The lipoic acid application creates a more distinct normalizing effect on the glutathione level. References
|