J.ophthalmol.(Ukraine).2015;6:37-41.
|
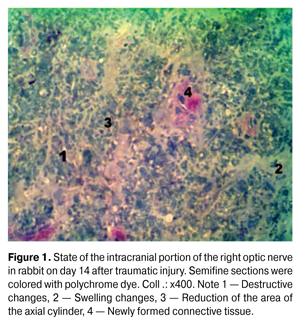
https://doi.org/10.31288/oftalmolzh201563741 Morphofunctional aspects of restorative processes in the optic nerve after traumatic injury under the influence of high doses of corticosteroids N.M. Moiseenko, PhD in Medical Sciences State Higher Education Institution “Ivano-Frankivsk National Medical University”, Department of Otolaryngology and Ophthalmology Ivano-Frankivsk (Ukraine) E-mail: natalymoyseenko@ukr.net Keywords: traumatic injury of the optic nerve, regenerative processes, remyelination, high doses of corticosteroids, the amplitude of the pupillary reaction. Search of conditions conducive to the activation of regenerative processes and restoration of the functions of the optic nerve after traumatic injuries is relevant. The purpose of this research was to study morphological and functional aspects of the rehabilitation process in the optic nerve after traumatic injury under the influence of high doses of corticosteroids. Material and methods. 24 adult rabbits were used,12 of them were from the group of rabbits injured without treatment and another 12 were treated with methylprednisolone on the second day after the injury. Morphological study was conducted on the 14th day, and the functional one (the amplitude of pupillary reaction) was carried out on the 14th day and in 1 month. Results After the use of high doses of corticosteroids in the intracranial portion of the optic nerve, on the 24th day, improvement of microcirculation, decreasing swelling and remyelination of nerve fibers were detected. At the same time, the amplitude of the pupillary reaction has increased in one month. Conclusions. It was established that in case of traumatic injury, usage of high-dose corticosteroids contributes not only to morphological but also to functional signs of activation of regenerative processes of the optic nerve. Introduction The opportunities for the optic nerve regeneration have been searched for decades. It is believed that retinal ganglion cells naturally are not able to regenerate the optic nerve after the injury. In particular, in 2004 it was widely thought that an imbalance between inflammatory and anti-inflammatory factors in the damaged optic nerve prevents its recovery [14]. However, subsequent studies revealed a number of factors that could provide it. Thus, according to Ivanova N, regenerative processes in the eye occur in three stages: alteration, changes in circulation and proliferation [2]. By using apoptosis inhibitors and growth factors, it has been possible to reduce a loss of retinal ganglion cells after the optic nerve injury [10, 15]. The use of glucocorticoids, which in high doses (30 mg/kg) promote the development of personal protective factor of ganglion cells, ensuring their survival is considered the most effective way of direct neuroprotection in traumatic lesions of the optic nerve [11]. Therefore, the use of high doses of corticosteroids is considered as a standard of treatment for traumatic optic neuropathy. If the searches for morphological base of regenerative processes of the optic nerve, despite still being debatable, have scientific rationale, the reconstituted fibers functioning is still doubtful and requires a more detailed study. Purpose: to study morphological aspects of restorative processes in the optic nerve after traumatic injury under the influence of high doses of corticosteroids. Material and methods Twenty four mature male rabbits, weighing 3.5-4 kg, breed Soviet Chinchilla, the optic nerves of which were injured in the operating room conditions were used as an experimental model [4]. Twelve of them received no treatment (injured group) and the other 12, on the second day after the injury, were treated (treated group) using i. m. administration of methylprednisolone in a dose of 30 mg / kg during 3 days, then the dose was gradually reduced. The control group consisted of 12 rabbits. In 2 weeks, a part of animals (6) from the both groups was withdrawn from the experiment via the guillotine. For morphological confirmation of damage, cranial portion of the optic nerve in both eyes was observed using light and electron microscopes. The corresponding structures of the control group were used as control ones. Surgery and morphological studies were conducted [4] in the operating room of vivarium of the Department of Human Anatomy of Ivano-Frankivsk National University in compliance with the rules of asepsis. Animal management and withdrawal from the experiment were carried out in accordance with the “Requirements of bioethics under the Declaration of Helsinki on ethical principles of medical research”. We determined the heart rate and the amplitude of the pupillary reactions (the difference between the diameter of the pupil, measured with a ruler in the horizontal meridian between 3 and 9 o'clock at twilight illumination and under direct light-striking with flashlight with a light power of 5W) on the 14th day (all animals) and in 1 month (6 animals from experimental groups) after injury. Computer data processing was performed using the statistical package Stat. Soft. Inc; Tulsa, OK, USA; Statistica 6. Results According to morphological studies on light-optical level of the intracranial portion of the optic nerve of rabbits from injured group on the 14th day (Fig. 1), significant swelling and destructive changes of the sheaths of myelinated nerve fibers (MNF) were observed, which led to a reduction of axial cylinders that were not detected in some nerve fibers, and the myelin sheath (MS) of these fibers occupied almost the entire area of the fiber. In endo- and perineurium the proliferation of connective tissue by newly formed collagen fibers was observed.
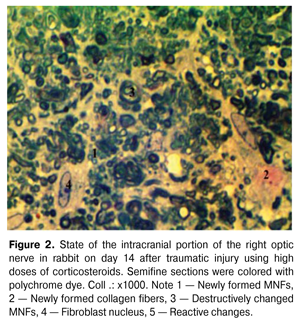
At the same time, a reduction of the swelling of endoneurial connective tissue has been observed after the treatment (Fig. 2). MNFs acquired a rounded or oval and irregular stellate and polygonal shape. In some MNFs, the external and internal contours of MS were concentric; in other ones, the inner layers of myelin formed protrusions of different shapes and heights, which is an evidence of the preservation of degenerative changes in these MNFs. [7]
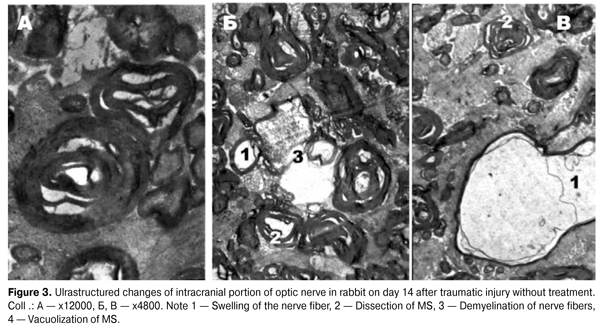
According to morphometry, a decrease in MNF area (p = 0.006972) alongside with a statistically significant increase in the g index (p = 0.000423) in intracranial portion of the right optic nerve, has been observed in the treated group compared to the injured one, indicating active processes of remyelination of these fibers. The electron-diffraction patterns of optic nerves in the group without treatment (Fig. 3) show that fibers’ axoplasm had a significant swelling of the MS of low electron density, and was vacuolated. The mitochondria were almost absent, and those that remained were swollen with enlightened matrix, deformed and partially destroyed by cristae. Strips of myelin were often chaotic, moving away from each other, having a wavy motion. All this, according to the studies, may indicate a disruption of axonal transport along the axial cylinders [6, 16, 17], caused by swelling of MS and can be interpreted as a periaxonal degeneracy. MNFs were often observed with symptoms of demyelination, specific manifestations of which was a deep disruption of the myelin sheath and a separation of myelin fragments that were freely located in the axoplasm or the cytoplasm of Schwann cell, indicating a disruption of the energy and protein metabolism [5, 8, 19].
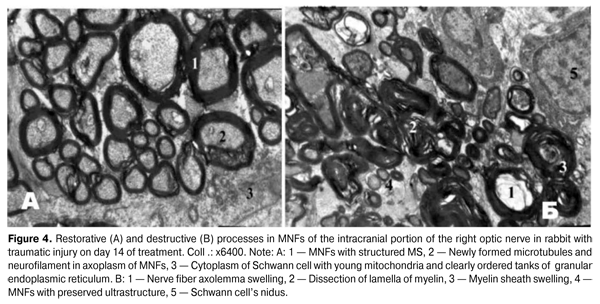
MNFs, the MS of which consisted only of several myelin lamellas, being in clear order have been detected in the animals from the treated group (Fig. 4 A). Axoplasm of such MNFs contained young elongated mitochondria with dense matrix and ordered cristae, moderate amount of clearly structured microtubules and neurofilaments, indicating a recovery of their backbone and conductivity [12]. In the Schwann cells’ cytoplasm, it has been discovered a large nidus with multiple dispersed chromatin and clear nucleolemma with shallow invaginations, young mitochondria and clearly ordered tanks of granular endoplasmic reticulum, having a large number of ribosomes located on their surface. These morphological features are evidence of active energy supplying and biosynthetic processes that occur in Schwann cells [7].
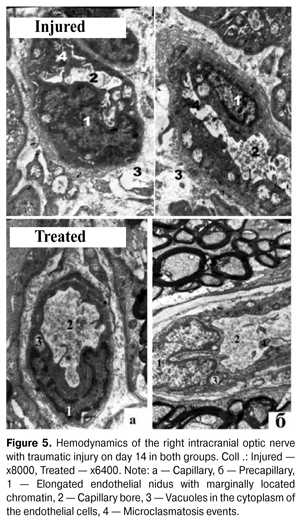
Such changes in the morphological structure of the treated group occurred against the background of microcirculation restoration. Endoneurium capillaries had rounded shape with oval or rounded clearance. The endotheliocyte niduses became elongated with marginally located chromatin (Fig. 5). The cytoplasm in endotheliocytes had an increased electron optical density and contained young mitochondria and single micropinocytotic vesicles, i.e. young endothelial cells [10].
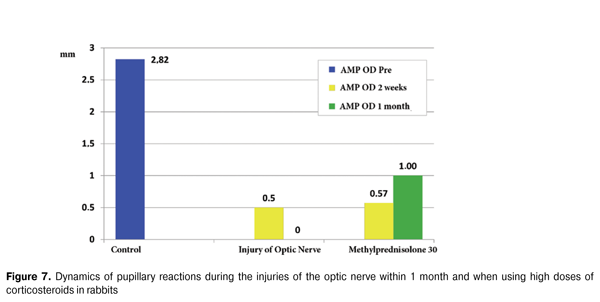
Regarding the functional activity, according to the pupillary reactions data, a complete lack of direct reaction to light on the affected side (amplitude was 0 mm) (Fig. 6) has been observed in the injured group. In contrast, within 1 month after the injury, a nonsignificant increase in the amplitude of the pupillary reaction to (1 ± 0,5) mm was observed in the treated group, indicating an activation of the afferent link of the optic tract and, possibly, a partial restoration of the functional ability of nerve fibers (Fig . 7).
Thus, on the 14th day after the use of high doses of corticosteroids, an improvement of microcirculation, a reduction of swelling and a remyelination of nerve fibers were discovered in the intracranial portion of the optic nerve. Along with this, amplitude of the pupillary response has increased in one month, indicating a recovery of functional activity of the optic nerve. However, despite a direct neuroprotective effect of high doses of corticosteroids, this method of treatment has a strong negative effect in connection with its high toxicity and mortality of experimental animals in the both groups in terms of 1 month. Conclusion Consequently, it has been established that the use of high doses of corticosteroids after the traumatic injury of the optic nerve contributes to the appearance not only of morphological but also of functional features of recovery processes activation in the optic nerve. References
|