J.ophthalmol.(Ukraine).2015;6:23-28.
|
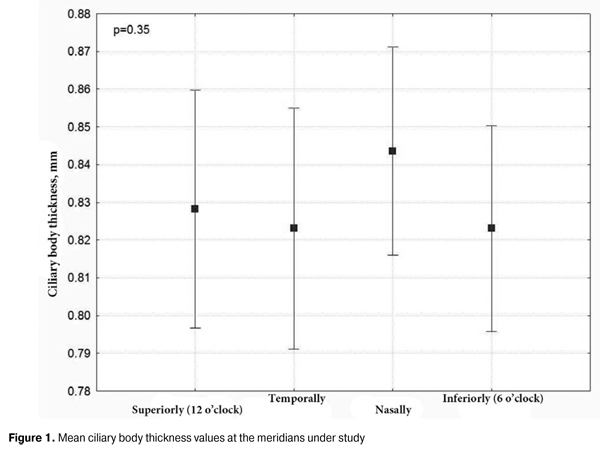
https://doi.org/10.31288/oftalmolzh201562328 Changes in ciliary body thickness over time following preoperative anti-inflammatory treatment in rhegmatogenous retinal detachment complicated by ciliochoroidal detachment G.V. Levitskaya, Cand. Sc. (Med) Alibet Yassine, Postgraduate Student N.V. Pasyechnikova, Dr. Sc. (Med), Prof Filatov Institute of Eye Diseases and Tissue Therapy Odessa (Ukraine) Е-mail: g.levytskaya@mail.ru Keywords: rhegmatogenous retinal detachment, retinal detachment, ciliochoroidal detachment, ciliary body edema, triamcinolone acetonide, perfluoropropane. Background: Rhegmatogenous retinal detachment (RRD), if complicated by ciliochoroidal detachment (CCD), is accompanied by marked hypotony and intraocular inflammation. However, to the best of our knowledge, there is no literature on the state of the ciliary body, its role in the development of this pathology, and specificity of the changes in it following the treatment aimed at resolving concomitant inflammation and choroidal attachment. Purpose: To assess the anatomical position and thickness of the ciliary body and to investigate the changes in the latter over time following anti-inflammatory pre-vitrectomy treatment in RRD complicated by CCD. Materials and Methods: Forty-three patients (43 eyes) with RRD complicated by CCD underwent standard ophthalmological examination (including assessment of the level of visual acuity, biomicroscopy, ophthalmoscopy and ocular tonometry) and ultrasound biomicroscopy of the ciliary body, choroid and retina both before and following anti-inflammatory pre-vitrectomy treatment. Results: At the baseline, all subject eyes had ciliary body edema and ciliary body detachment extending into the choroid. In ciliary body detachment, the morphological features of the ciliary body included ciliary body edema and disorganization of the supraciliary layer of the pars plana, which was evident by the presence of multiple small oblique fibers. In all subject eyes, the treatment resulted in the achievement of reattachment of the choroid and the ciliary body as well as a reduction in ciliary body edema (total mean ciliary thickness reduced from 0.83 ± 0.09 to 0.65 ± 0.09 mm, with a difference of 0.18 ± 0.07 mm, P < 0.001). Conclusion: Preoperative anti-inflammatory treatment in RRD complicated by CCD resulted in restoration of the anatomical position of the ciliary body and a statistically significant reduction in ciliary body edema. Introduction The prognosis for rhegmatogenous retinal detachment (RRD) complicated by ciliochoroidal detachment (CCD), marked hypotony and intraocular inflammation is rather unfavorable, which is confirmed by poor anatomical and functional outcomes of the treatment and by a high rate of redetachment [1, 2]. Therefore, this form of retinal detachment is usually an exclusion criterion in multicenter prospective studies of the efficacy of treatment of RRD [3]. It has been reported that administration of oral steroids (prednisolone, 1 mg per kg) before primary vitrectomy in eyes with combined RRD and choroidal detachment improves reattachment rates [4]. The preoperative pretreatment of such eyes involving intravitreal injections of triamcinolone acetonide, either combined with expanding gases in especially severe cases [5], or alone [6] has a number of advantages. The aim of preoperative treatment of such eyes is resolution of choroidal detachment and signs of concomitant intraocular inflammation. Anti-inflammatory treatment has been shown also to result in a significant decrease in hypotony [4-8], thus evidencing the restoration of the function of the ciliary body. The literature is, however, scant on the features of morphological changes in the ciliary body in combined RRD and CCD [9-10], and, to the best of our knowledge, the changes in the state of this body over time following anti-inflammatory treatment has not been investigated. The purpose of the study was to assess the anatomical position and thickness of the ciliary body and to investigate the changes in the latter over time following anti-inflammatory pre-vitrectomy treatment in RRD complicated by CCD, uveitis and hypotony. Materials and Methods The prospective study involved forty-three patients (20 men and 23 women; 43 eyes; age, 24 to 83 years) with RRD complicated by concomitant CCD and intraocular inflammation. Exclusion criteria were a history of previous ocular inflammatory diseases, ocular trauma or retinal surgery. All patients underwent standard ophthalmological examination including assessment of the level of visual acuity, biomicroscopy, ophthalmoscopy and ocular tonometry. Additionally, ultrasound biomicroscopy (UBM) of the ciliary body, choroid and retina was performed. Marked hypotony was the feature of the clinical course of retinal detachment in these patients, with a mean intraocular pressure (IOP) level of 6.7 ±1.5 mm Hg (range, 5 to 11 mm Hg). Choroidal detachment in three or more quadrants was found in 65.1% of cases, with a mean height of the detachment of 4.12 ± 2.18 mm (range, 0.3 mm to 8.5 mm). Ciliary body thickness measurements were made under cycloplegia with phenylephrine hydrochloride 10% and cyclopentolate hydrochloride 1%, between the ciliary processes located most closely to the scleral spur at four meridians (12 o’clock, 6 o’clock, nasally (3 o'clock OD and 9 o'clock OS), and temporally (9 o'clock OD and 3 o'clock OS)). We used the Aviso UBM unit (Quantel Medical) with a 50-MHz linear probe (axial resolution: 35 µm; lateral resolution: 60 µm). Examination was performed with the patient lying on his back, and with the head of the bed raised. Prior to vitrectomy, under above-mentioned cycloplegic conditions, eyes of the study received anti-inflammatory therapy, with 4 mg of (0.1 mL) triamcinolone acetonide intravitreally injected either alone (27 eyes) or in combination with 0.4 to 0.8 mL of perfluorpropane until normalization of IOP (16 eyes). This treatment aimed at preoperative resolution of choroidal detachment and intraocular inflammation to decrease the risk of intra- and postoperative complications in retinal detachment surgery. Ultrasound biomicroscopy was performed both before and 1 to 4 days after intravitreal injection. Statistical analysis was performed with the use of STATISTICA-8 software. If there was a normal distribution, a parametric t test was used. The level of significance p ? 0.05 was assumed. Data are presented as mean and standard deviations (SD) [11]. Results Baseline UBM revealed ciliary body edema with detachment and extending from the ciliary body into the choroid in all patients. It is noteworthy that in this category of patients, ciliary detachment was characterized by the detachment of the pars plana only, whereas the attachment of the pars plicata to the sclera was maintained. Therefore, no connection was observed between the subchoroidal spaces and the anterior chamber, which is a feature that distinguishes the ciliary detachment from other pathology in the eye with RRD. The choroidal detachment in all subject eyes was characteristic in that it extended to the equator or a little posterior to the equator, without involvement of the posterior pole. The maximum height of the choroidal detachment was observed in cases with the maximum extent of this detachment. The mean ciliary body thickness at the above-mentioned four meridians varied from 0.82 mm to 0.84 mm, with the SD of 0.01 to 0.02. Additionally, no significant difference was revealed between the mean ciliary body thickness values at different locations, P = 0.35 (Fig. 1).
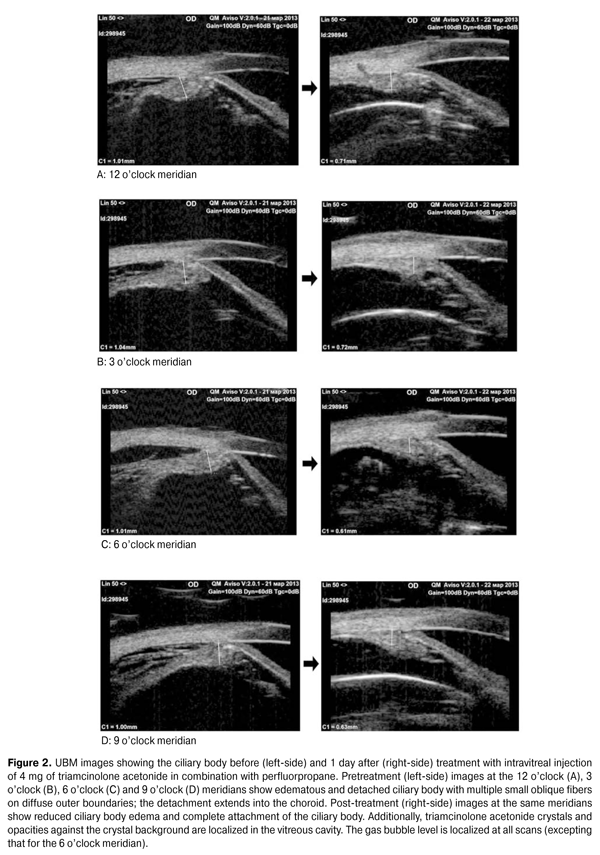
Therefore, it is possible to assess changes in the ciliary body thickness based on the total mean value of this index at different meridians for all subject eyes, which was 0.83 ± 0.09 (range, 0.68 to 1.05 mm; median, 0.82 mm) in the study reported here. Resolution of intraocular inflammation, reduction in choroidal detachment and normalization of the IOP were the basic criteria for the efficacy of preoperative treatment. One to two days following an intravitreal injection, no signs of anterior uveitis (ciliary tenderness, conjunctival injection and posterior synechiae) were found in any patient. One to four days following an intravitreal injection, the IOP increased from baseline of 6.7 ± 1.5 to 13.4 ± 0.9 mm Hg (P = 0.0001), indirectly evidencing an improvement in the function of the ciliary body (and particularly restoration of intraocular fluid production). Visual acuity, a conventional criterion for the efficacy of treatment, was not appropriate in our study, since preoperative treatment was just to create conditions for uncomplicated vitrectomy, and the retina was not attached at that stage. Postoperative ultrasound biomicroscopy was performed in the presence of a reduction in the signs of intraocular inflammation and partial restoration of normal IOP levels and revealed that ciliary body and choroidal re-attachment as well as a reduction in ciliary body edema was achieved in all 44 subject eyes. The pars plana thickness was also measured at four points (superiorly (12 o’clock), inferiorly (6 o’clock), nasally (3 o'clock OD and 9 o'clock OS), and temporally (9 o'clock OD and 3 o'clock OS)) (Fig. 2).
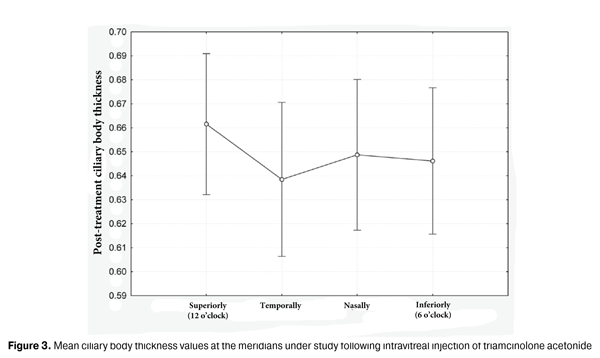
It is noteworthy that re-attachment of the ciliary body and choroid was achieved at all the above-mentioned meridians (i.e., in the inferior globe also rather than only at the 12 o’clock meridian, in the area lying close to gas bubble pressure) with the patient’s head positioned vertically. This finding confirms that our approach to the treatment of this specific form of RRD is justified pathogenetically. Moreover, in all subject eyes, UBM revealed a reduction in the ciliary body edema following intravitreal injection of triamcinolone acetonide. Given no statistically significant difference in the ciliary body thickness at the four major meridians, the changes in the thickness values over time were assessed based on the mean thickness values (Fig. 3). The mean index was 0.65 (0.09) mm (range, 0.51 mm to 0.93 mm; median, 0.63 mm).
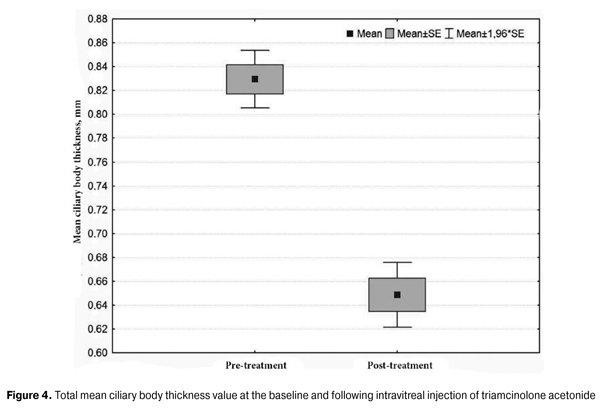
Therefore, the total mean ciliary body thickness value changed significantly from baseline following treatment, evidencing a significant reduction in ciliary body edema (baseline 0.83 (0.09) mm vs post-treatment 0.65 (0.09) mm, with a difference of 0.18 (0.07) mm; P < 0.001) (Fig. 4).
Consequently, it can be argued that preoperative anti-inflammatory pretreatment in combined rhegmatogenous retinal detachment and ciliochoroidal detachment results in re-attachment of the choroid and the ciliary body. A reduction in ciliary body edema and return of ciliary body function with an improvement in ocular hydrodynamic indices was a sign confirming resolution of concomitant intraocular inflammation. The therapeutic effect obtained in the study advocates one of the theories of the pathogenesis of the development of ciliochoroidal detachment. The mechanism of the development of ciliochoroidal detachment in ocular trauma and in a complicated glaucoma surgery has been described in details in [12, 13] and [14], respectively. However, the development of ciliochoroidal detachment in RRD follows another mechanism, which has been significantly less studied. Jarret [10] has reported a hypothesis regarding the development of ciliochoroidal detachment in RRD more completely than others; his paper [10] involved the analysis of 47 relevant cases found during a 12-year observation. RRD is known to be accompanied by a blood ocular barrier breakdown [15, 16]; it is the latter that is a casual event of increased vascular permeability, transudation or exudation of fluid into the extracellular spaces, accumulation of fluid in the suprachoroidal spaces, and ciliary body and choroidal detachment. This in turn results in decreased aqueous production and development of acute hypotony, thus completing the vicious cycle [10]. An increased absorbing surface of the retinal pigment epithelium exposed to the subretinal fluid is another cause for the presence of marked hypotony in RRD [17]. This might explain the fact that in our study, the IOP level was restored incompletely (just to 13.1 ± 0.8 mm Hg) despite successful re-attachment of the ciliary body and the choroid. Seelenfreund M.H et al. [18] believe that the height and extent of ciliochoroidal detachment might depend on the degree of vitreous contraction and state of choroidal vessels. We find this hypothesis likely; it explains why CCD is more often found in elder RRD patients than in those of other age groups. We have failed to find the literature on the morphometric features of the ciliary body in combined ciliary body detachment and RRD. It has been reported that following scleral buckling surgery for RRD the findings on the ciliary body-related changes [19] involved ciliary edema which was in some cases accompanied by increased IOP levels [20] due to alterations in the anterior chamber angle within 3 days after surgery [20-21]. These alterations are caused not only by postoperative edema, but also by the presence of sclera buckling material which displaces the ciliary body anteriorly. In the following days, as inflammation subsided, approximately by day 28, the initial ciliary body thickness and ocular hydrodynamic indices restored [19]. Conclusion Preoperative anti-inflammatory treatment in RRD complicated by CCD resulted in restoration of the anatomical position of the ciliary body and a statistically significant reduction in ciliary body edema. The mechanism of the development of ciliochoroidal detachment in the presence of RRD is likely to be based on a blood ocular barrier breakdown, which explains a high efficacy of the treatment used. References
|