J.ophthalmol.(Ukraine).2015;6:46-49.
|
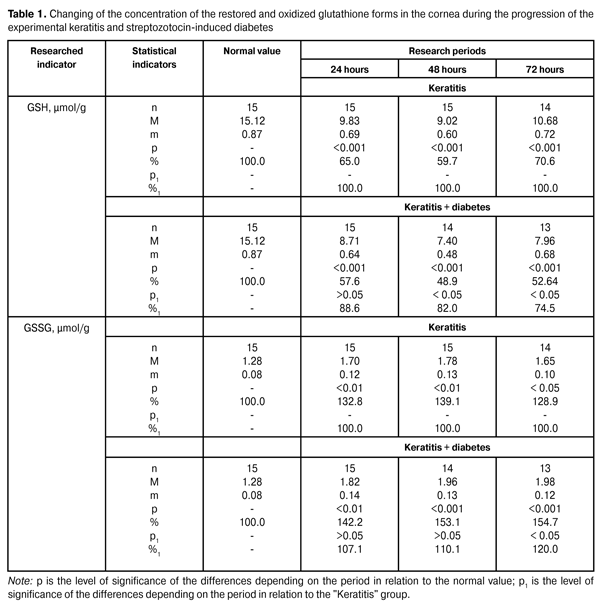
https://doi.org/10.31288/oftalmolzh201564649 Study of the thiol system redox potentials in the cornea in experimental keratitis on the background of diabetes progression T.M. Zhmud, PhD in Medical Sciences Vinnitsa State Pirogov Memorial Medical University (Vinnitsa, Ukraine) E-mail: Gtatyana@email.ua Keywords: keratitis, streptozotocin-induced diabetes, restored glutathione, oxidized glutathione. Introduction. Researches of the pathological states of the surface tissues of the eyeball to date remain relevant. Objective. To study the state of the thiol system redox potentials in the cornea in experimental keratitis on the background of diabetes progression Material and methods. To conduct the experiments 52 rabbits were used. They were divided into three groups: the first was the control group (8 rabbits), the second was the experimental group of animals with keratitis (23 rabbits), the third was the experimental group of animals with keratitis on the background of the streptozotocin-induced diabetes (22 rabbits). In the corneal tissue the definition of reduced and oxidized glutathione was performed. Results. In conditions of the progression of experimental keratitis and streptozotocin-induced diabetes, the reduction of the restored glutathione level and the increase of the oxidized glutathione level in the tissue of the cornea were determined. Conclusions. 1. Under the conditions of keratitis progression, the glutathione status disruption in the tissues of the cornea is expressed in animals with diabetes more significantly. The level of the restored glutathione was reduced comparing to animals with keratitis without non-streptozotocin-induced diabetes by 11.4 % during the first period, and by 18.0 % during the second period, and by 25.5 % during the third period. 2. In the progression of the experimental keratitis and streptozotocin-induced diabetes, the increase of oxidized glutathione in the cornea was observed. In different observation periods in such conditions, the concentration of the oxidized glutathione in the cornea has increased by 7.1%, 10.1% and 20.0%. Introduction Today, the keratitis are a serious problem in ophthalmology, because of their degree of incidence, predisposition to the process chronization, difficult treatment and often severe sequelae, such as perforation of the cornea, cataracta complicata, secondary glaucoma, optic nerve neuritis, endophthalmitis etc. [2]. In recent times, keratitis etiology and pathogenesis remain poorly researched, that causes a lack of highly effective methods of their treatment. The use of traditional treatment methods is not always resulting in patient’s recovery and prevents the occurrence of relapses. Therefore, the search of new methods of pathogenetic action in keratitis remains relevant [3]. Corneal opacity is observed in patients with keratitis. It develops as a result of cornea infiltration, which is followed by transparency and gloss decrease, sphericity and sensitivity violation [13]. In a number of studies the role of surface eye structures was revealed in connection with protective and adaptive reactions of the visual organ. Also, a new functional feature of the conjunctiva, which is associated with the transportation of important glutathione detoxicant, was discovered [8, 9, 11]. Glutathione performs all the protective functions in its reduced form. Oxidized glutathione cannot function in the reactions of eye tissue protection. And if it is not restored by glutathione reductase, it diffuses out of the tissues freely, which leads to a decrease in the general level of glutathione in the lens [6, 7]. Due to this functional feature of the conjunctiva, tears include a significant amount of glutathione and its concentration in this biological fluid exceeds its level in the blood plasma. Certainly, a high level of glutathione in tears considering its functional indicators allows tears to represent a strong protective barrier for eye tissues and especially for the cornea. Restored glutathione through its own oxygenation recovers and neutralises hydrogen peroxide, as well as organic hydroperoxides, participates in the processes of detoxification, provides stability of the protein and lipid structures of cell membranes [7]. In providing an intracellular balance of glutathione redox system, the thiol status plays an important role. It determines the rate of thiol groups’ oxidation under the influence of oxidative stress in the tissues and biological liquids of the eye. Integral indicator of the thiol status is the level of free sulphide groups and glutathione. At this time, the researches were conducted, in which it was revealed that the development of the keratitis violates strongly glutathione status in the tissues of the cornea. It has been observed in animals with allergic conjunctivitis. In the last case, the level of the restored glutathione was relatively reduced by 25% [1]. Keratitis in animals with allergic conjunctivitis was followed by significant damage of redox processes in the cornea. The activity of the cytosolic enzyme — lactatdehydrogenase — was reduced by 14.3% during the first observation period and the mitochondrial enzyme activity indicators (malate dehydrogenase) were reduced by 17.6% during the second observation period in animals with keratitis in the background of conjunctivitis compared with a group where keratitis was progressing without the inflammatory process in the conjunctiva [1]. Particularly promising in this direction is the status of the thiol system in the cornea during the inflammatory diseases of the anterior part of the eye in the conditions of diabetes progression. As a result of the research, it was found that during the development of the streptozotocin-induced diabetes, a sharp decrease of the reduced potential of the glutathione system is noted, primarily due to a sharp decrease in the level of the reduced form of the coenzyme [6, 12, 13]. It was also shown that in the study of various forms of glutathione in patients with initial forms of diabetic retinopathy, the level of its restored form lowered by 60%, and the enzymatic and non-enzymatic potential of the antioxidant system in the retina — restored glutathione level — lowered significantly [5]. To date, there is no data on the changes in glutathione status in the cornea during the experimental keratitis against the background of experimental diabetes. The objective of the work is to study the status of thiol system redox potentials in the cornea in experimental keratitis on the background of diabetes progression. Research material and methods To conduct the experiments, 52 Chinchilla rabbits weighing 2.2-2.9 kg were used. Experimental animals were divided into three groups: the first was the control group (8 rabbits), the second was the experimental group of animals with keratitis (23 rabbits), and the third was the experimental group of animals with keratitis on the background of the streptozotocin-induced diabetes (21 rabbits). During the experiment the recommendations on animal researches, adopted by the international society in relation to vision organ studies were followed. Experimental keratitis in animals was caused by the intrastromal injection of 50 µL 0.2% endotoxin lipopolysaccharide on phosphate buffer [10]. Diabetes was caused by injecting streptozotocin (55 mg per 1 kg of body weight, intraperitoneally). Insulin was administered to the diabetic animals to prevent the weight decrease provided hyperglycaemia maintenance (blood sugar levels ranged from 20 to 25 µmol/L). Restored and oxidized glutathione were determined in the tissue of the cornea in the terms: 24, 48 and 72 hours. To determine the restored glutathione, a tissue homogenate was prepared with the use of 6% chloric suspension in the ratio of 1:10 (tissue weight: volume of the medium for the homogenisation). After the centrifugation at 5?C during 10 minutes at 1000 rpm, the supernatant fluid was neutralised with 1.75 M solution of tri-basic potassium phosphate. In the resulting neutral extract the content of the restored glutathione (µmol/g) of the tissue was determined using an enzymatic method. The principle of the method for determining the oxidized form of glutathione consists in that as a result of enzymatic restoration of glutathione by glutathione reductase, the oxidation of the restored form of nicotinamide-adenine dinucleotide phosphate (NADFN2) takes place, the lowering of which is registered with a spectrophotometer at a wavelength of 340 nm. Course of work. The homogenate was prepared in the ratio of 1:10 (tissue weight: volume of the medium for the homogenisation). After completing of definition of the thiol form of glutathione content, 0.1 ml of 11 µmol/L NADPN solution was added into the same cuvette. We stirred and recorded the optical density (E4) at a wavelength of 340 nm. We added 0.01 ml of glutathione reductase suspension (0.018 Un/ml of the reaction solution) and recorded the optical density of the solution after having completed the reaction (E5). The range of determined levels of the restored and oxygenised forms is from 5 to 200 µg/ml of relevant solution. The average value of the variation coefficient for the specified range of the restored form is 4.0%, and 5% for the oxygenised form. The measurements were performed using spectrophotometer SF-26 with the optimal interval 0.1-0.5 of working range on the optical density scale. Content of the oxygenised glutathione was measured in µmol/g of the tissue. The data obtained were subject to the statistical processing by using the SPSS 11.0 package [4]. Results and their discussion Data on the concentration of the restored and oxygenized glutathione forms in the cornea during the progression of the experimental keratitis and streptozotocin-induced diabetes are presented in Table 1.
When determining the level of the restored glutathione form in the cornea of animals with diabetes progression it was found that its indicators lowered to (11.64 ± 0.84) µmol/g, that amounted to 77.0% compared with its normal value (15.12 ± 0.87) µmol/g (p<0.01). As it is evident from the presented data, the level of the restored form of glutathione in the cornea of animals in the group with keratitis decreased to 65.0%, which amounted to (9.83 ± 0.69) µmol/g in the 1st period (p <0.001), to 59.7%, which is equal to (9.02 ± 0.60) µmol/g in the 2nd observation period (p <0.001), and to 70.6%, being equal to (10.68 ± 0.72) µmol/g in the 3rd period compared with the normal value (p <0.001). The data presented in Table 1, show that the level of the restored glutathione in the cornea of animals with keratitis and diabetes lowered in the 1st period to 57.6%, i. e. (8.71 ± 0.64) µmol/g compared to the normal value (p < 0.001). During the 2nd period this indicator lowered to 48.9% (7.40 ± 0.48) µmol/g with respect to the normal value (p < 0.001), and in the 3rd period it lowered to 52.6%, amounting to (7.96 ± 0.68) µmol/g compared to the normal value (p < 0.001). When comparing the data of two research groups, we can note that the level of the restored glutathione in the cornea of animals with keratitis and experimental streptozotocin-induced diabetes was lowering to a greater extent compared with the data of animals with keratitis caused without diabetes. Thus, in the 1st period the lowering amounted to 11.4%, in the 2nd period – to 18% (p < 0.05) and in the 3rd period – to 25.5% (p < 0.05). It should be noted that the level of oxidised glutathione form in the cornea of animals with diabetes progression increased to (1.57 ± 0.11) µmol/g, that was 122, 7% compared to the normal value (1.27 ± 0.08) µmol/g. As a result of the studies performed it has been shown that the level of oxidised form of glutathione in the cornea of animals with keratitis has increased to 132.8% (1.70 ± 0.12) µmol/g in the 1st period (p < 0.01), to 139.1% (1.78 ± 0.13) µmol/g in the 2nd observation period (p < 0.01) and to 128.9% (1.65 ± 0.10) µmol/g in the 3rd period compared to the normal value (p < 0,05). The level of oxidised glutathione in cornea of animals with keratitis and diabetes increased to 142, 2% (1.82 ± 0.14) µmol/g in the 1st period compared to the normal value (p < 0.01). In the 2nd period this indicator increased to 153.1% that amounted to (1.96 ± 0.13) µmol/g compared to the normal value (p < 0.001). In the 3rd period it has been noted an increase to 154.7%, i. e. (1.98 ± 0.12) µmol/g in the level of oxidised glutathione, i. e. (1.98 ± 0.12) µmol/g compared to the normal value (p < 0.001). It should be noted that in all observation periods in animals with keratitis with diabetes progression, an increase of oxidised glutathione concentration was noted, compared to the group of animals with keratitis and without diabetes. Thus, during the 1st period the increase was 7.1%, in the 2nd period it was 10.1%, and in the 3rd period it was 20.0%. Summarizing the data obtained in the study on the redox potentials of the thiol system in the cornea with experimental keratitis on the background of diabetes progression, it is necessary to note a significant violation of restored glutathione system potential in the tissue of the cornea. In conditions of keratitis on the background of the diabetes this violation was of more abrupt character than in animals with keratitis without diabetes. Conclusions 1.It was found that under the keratitis progression the glutathione status violation in the cornea tissues is more expressed in animals with diabetes, the level of the restored glutathione was lowered compared to the group of animals with keratitis without diabetes to 11.4% in the 1st period, to 18,0% in the 2nd period and to 25,5% in the 3rd period. 2.In the progression of the experimental keratitis and streptozotocin-induced diabetes, the increase of oxidized glutathione in the cornea was noted. In different observation periods in such conditions, the concentration of the oxidized glutathione in the cornea has increased by 7.1%, 10.1% and 20.0%.
References Haydamaka Т.B., Rafalyuk S.Ya. Influence of endotoxin-induced keratitis on the redcingpotential of glutathione in the cornea of animals with experimental syndrome of the dry eye // Ophthalmol Zh. 2014;6:72 – 77. Russian. Drozhzhina G.I. Viral desease of the cornea and conjunctiva. Zdorovya Ukrainy. 5.;2002:35-36. Russian. MaichukYu.F. Farmacotherapy of inflammatory diseases of eyes : yesterday, today, tomorrow // Material sofresearc hand practice conference of November, 20 - 21, 2001 the "Pressing questions of inflammatory diseases of eyes". M.; 2002. 7–17. Russian. Nasledov А. SPSS computer analysis of data in psychology and social sciences. Spb.: Piter; 2005. 416 p. Oleinik Т. В. Modern pathogenetically oriented ways of prophylaxis and treatment of early stages of diabetic retinopathy; thesis for Doctorof Med. Scince. Donetsk; 2010. 268 p. PetrunyaA.M., Muhamed Kutaini. Research of thiol exchange and ORP processes in a cornea at the experimentalc onjunctivitis. Problemy ekological and medical geneticists and clinical immunology. 2012;1 (109):259-272. Russian. Selivanova О. V. Clinical and experience ground of correction of level of thiol connections in tissue of conjunctiva and tear liquid at medicamental treatment of conjunctivitis: thesis for Candidate of Med. Scince:14.01.18 " Ophthalmology" Kyiv; 2011. 20 p. Semesko S.G. Clinical value of research of antioxidant status in ophthalmology. Vestn. Ophthalmol.2005;3: 44-47. Russian. Azar D. T., Spurr-Michaud S. J. Altered epithelial-basement membrane interactions in diabetic corneas. Arch. Ophthalmol.1992;110:537-540. Bergmeyer H. U. Methods of enzymatic analyses. 1984. 2220 p. Bilen H., Ates O., Astam N., Uslu H.,Akcay G., Baykal O. Conjunctival flora in patients with type 1 or type 2 diabetes mellitus. Adv. Ther. 2007;24:1028–1035. Chikama T, Wakuta M., Liu Y., Nishida T. Deviated mechanism of wound healing in diabetic corneas. Cornea. 2007;26 Suppl1: S75–S81. Citiric M, Berker N, Haksever H. Conjunctival impression cytology in non-proliferative and proliferative diabetic retinopathy. Int. J. Ophthalmol. 2014;7:321-325. Didenko T. N., Smoliakova G. P., Sorokin E. L., Egorov V. V. Clinical and pathogenetic features of neurotrophic corneal disorders in diabetes.Vestn. Oftalmol. 1999;115: 7–11. |