J.ophthalmol.(Ukraine).2015;5:46-51.
|
https://doi.org/10.31288/oftalmolzh201554651 Stabilizing quercetin and lipoate effect on enzymatic antioxidant system (glutathione peroxidase and glutathione reductase) in the retina during experimental diabetes treatment O. A. Moroz, Ph.D. in Medical Sciences A. Novak Transcarpathian Regional Clinical Hospital Uzhgorod (Ukraine) E-mail: moroz.oleg@gmail.com Introduction. The thesis actuality consists in studying the quercetin and lipoate effect during experimental diabetes treatment. Objective. To study the quercetin and lipoate effect on the antioxidant system enzymes, particularly, glutathione peroxidase and glutathione reductase during experimental diabetes. Material and methods. Researches were conducted on white rats. Experimental animals were divided into four groups: the first one – control group (14 rats); the second one – experimental group (14 rats), animals with developing diabetes, without drug administration; the third one – experimental group (12 rats), animals with developing diabetes and lipoic acid administration; the fourth one – experimental group (15 rats), animals with developing diabetes and quercetin administration. The activity of glutathione peroxidase and glutathione reductase was determined in homogenates of retinas and blood plasma. Results. The usage of thiol preparations and bioflavanoid considerably contributes to the stabilization of hydroperoxide neutralization processes in the retina in conditions of experimental diabetes. It is also required to specify that the effect of a thiol preparation – lipoic acid was more expressed as compared with quercetin. Conclusion. The effect of lipoic acid and quercetin preparations during the experimental diabetes (2 & 6 months) considerably allows avoiding the inhibition of antioxidant system enzymes in animal retina. It is confirmed by the increase of the activity of glutathione peroxidase in the retina when using lipoate by 65.0% (in 2 months) and by 74.3% (in 6 months), and glutathione reductase - by 26.7% (in 2 months) and by 34.4% (in 6 months) for the group of untreated animals. The quercetin injection increases the activity of glutathione peroxidase by 60.6% (in 2 months) and by 65.9% after six months, and glutathione reductase by 20.4 and 29.8% correspondingly. The most expressed effect for reducing the degree of damage of antioxidant system enzymes is specific for lipoic acid. Key words: streptozotocin-induced diabetes, retina, glutathione peroxidase, glutathione reductase, quercetin, lipoate, experiment
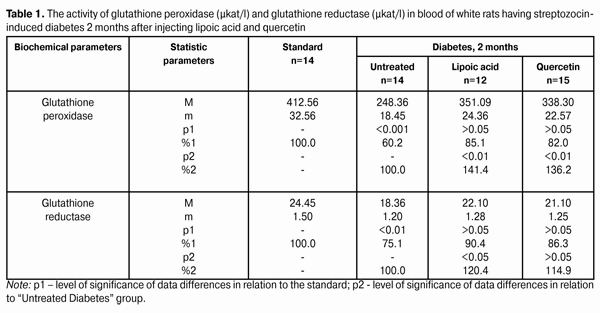
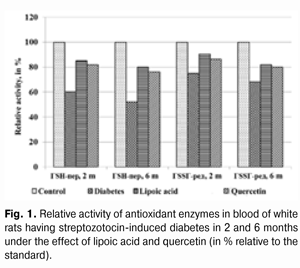
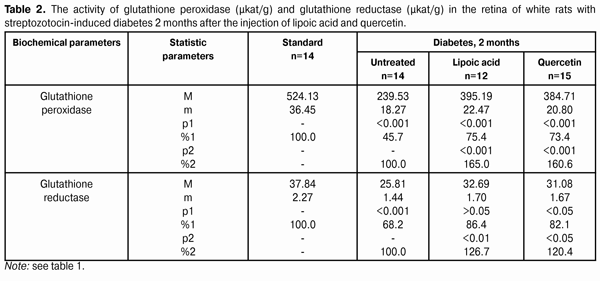
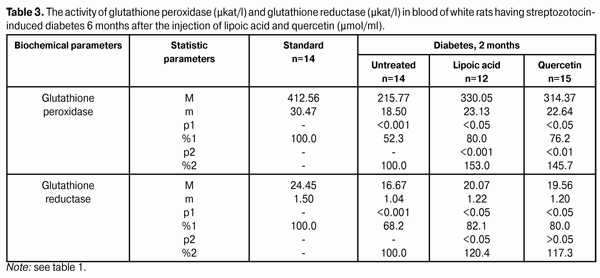
Introduction Diabetes mellitus (DM) occupies the third place among the direct death reasons in the world after cardiovascular and oncological diseases and it is the leader of complications occurrence causing the early disability of patients [2, 5, 6, 15]. Depending on the type and duration of diabetes mellitus course, the diabetic retinopathy progresses in practically all the patients; this is one of the main reasons of visual disability in able-bodied persons in economically developed countries. It requires the development of effective methods of diabetic retinopathy treatment that is possible only on the basis of in-depth study of nosotropic mechanisms of this disease [1, 3, 8, 11, 12, 21, 23, 25]. Some studies have demonstrated a satisfactory evidence of the role of free-radical processes and, particularly, lipid peroxidation (LPO) in the mechanism of diabetic damages of vases and other tissue structures [13, 14]. During DM, the LPO intensification occurs in the retina and pigmental epithelium by forming a redundant quantity of free radicals that causes a destabilization and a subsequent breakdown of cell elements. In this case, the highest LPO intensity is observed in the receptor retina layer. Afterwards, the soluble LPO products diffuse into inner retina layers and are found in the inner sympatic layer and ganglion cells layer [4, 17]. A high level of lipid peroxide is also determined in the blood plasma. In erythrocytes, the level of intermediate LPO product – malondialdehyde rises and progressively grows while the ocular fundus changes are increasing. The high activity of oxidative reactions during the DM directly correlates with the intensity of vascular complications. The progress of these reactions causes the death of endotheliocytes, lipids oxidation and their modification as well as the transition of platelets into the reactivity state [18, 20]. Therefore, it is required to find out a recovery mechanism of LPO processes with the help of glutathione system. In the meantime, it is demonstrated that during the streptozotocin-induced diabetes there was a sudden reduction of glutathione contents in the eye retina of experimental animals, mainly, by reducing the concentration of its recovered form. The stimulation of glutathione peroxidase reaction, neutralizing lipids hydroperoxides, which level during diabetes, as known, considerably rises, plays an important role in the mechanism of change of oxidized and reduced glutathione form ratio. It should also be considered that the reduced glutathione form can directly quench free-radical compounds, besides, a drop of general level of reduced glutathione can occur by forming a glutathione conjugate with hydroxyl aldehydes and other toxic compounds which in such a conjugated form are excreted from the organism [19, 22, 24]. According to the data of experimental studies, when the experimental diabetes progress, the activity of enzymes of the antioxidant system (superoxide dismutase, catalase and glutathione peroxidase) is progressively reduced. The application of В6 vitamin preparations in the initial period of experimental diabetes (2 months) considerably allows preventing the inhibition of antioxidant system enzymes. It has been noted that В6 vitamin had the most expressed effect on enzymes - glutathione peroxidase and superoxide dismutase [10]. It has been also determined that the application of complex of functionally conjugate co-enzymes considerably activates the enzymes of the antioxidant system (superoxide dismutase, catalase and glutathione peroxidase) in the retina of white rats in 28 days after the streptozotocin-induced diabetes development [9]. The more expressed stimulation with respect to glutathione peroxidase is caused by “Milgamma” and “Fakovit” preparations. Under the effect of these preparations, an activity rise of this enzyme is registered in the retina of animals during experimental diabetes (28 days) [9]. So the necessity of studying the state of enzymatic antioxidant system as well as of searching its stimulation methods during the treatment of diabetic retinopathy is obvious. Work objective: to study quercetin and lipoate effect on antioxidant system enzymes, in particular, on glutathione peroxidase and glutathione reductase during the experimental diabetes. Material and methods The investigations were carried on albino Wistar rats weighing 180 – 210 g. Experimental animals were divided into four groups: first – control group (14 rats), second – experimental group (14 rats), animals with developing diabetes, without drug administration, third – experimental group (12 rats), animals with developing diabetes and lipoic acid administration, fourth – experimental group (15 rats), animals with developing diabetes and quercetin administration. Quercetin and lipoic acid were administered to the animals with developing diabetes orally during the whole experimental period (6 months). During the performance of the experiment, the recommendations accepted by international community for visual organ study with regard to the investigations of animals were followed. The diabetes was induced by means of streptozotocin injection (55 mg per 1 kg of body weight, i.p.). Two months after the diabetes progress, some animals (separate groups), being in different experiment conditions as well as normal rats (control) were decapitated with thiopental sodium preanesthetic (50 preparation mg per weight kg). The eyes were enucleated on ice at a temperature of 0 – 5°С. Six months after the diabetes progress, the rest of animals, still being in different experiment conditions, were also pithed according to the appropriate rules. The deleted retina of animals came to study at once. The activity of glutathione peroxidase and glutathione reductase was determined in the retina homogenates and blood plasma. The activity of glutathione peroxidase was determined spectrophotometrically following the rate of oxidized glutathione formation with the help of conjugated reactions with the NADPN-dependent enzyme - glutathione reductase, by recording the change of optical density during NADPN oxidation. The optical density change was determined at the wave length of 340 nm during 1 min using “Specol-210” spectrophotometer. The enzyme activity was expressed in protein ?kat/g or blood plasma ?kat/l. The variation factor was 1.8% [16]. The activity of glutathione reductase was determined using spectrophotometric analysis method. The enzyme activity was expressed in protein ?kat/g or blood plasma ?kat/l [16]. The data obtained were statistically processed using SPSS 11.0 package [7]. Results and their discussion The data on the activity of glutathione peroxidase and glutathione reductase in blood of white rats having streptozotocin-induced diabetes 2 months after injecting lipoic acid and quercetin are specified in Table 1 and diagram (Fig. 1).
When applying lipoic acid, the activity of glutathione peroxidase in blood of animals rose to (351.09±24.36) ?kat/l, i.e. it rose by 41.4% (р<0.01) as compared with the untreated group - (248.36±18.45) ?kat/l. When quercetin was applied, the activity of glutathione peroxidase rose by 36.2%, while composing of (338.30±22.57) ?kat/l in relation to the group of untreated animals (р<0.01). Meanwhile the enzyme activity in the group of untreated animals having diabetes was reduced to 60.2% in relation to the standard (412.56±32.56) ?kat/l (р<0.001). The activity of glutathione reductase in blood plasma during the experimental diabetes progress and lipoic acid application rose to (22.10±1.28) ?kat/l that was 120.4% in relation to the group of diabetic untreated animals (18.36±1.20) ?kat/l (р<0.05). In the group of diabetic animals with quercetin administration, the activity of glutathione reductase rose by 14.9%, or to (21.10±1.25) ?kat/l as compared with the “Diabetes” animal group. Meanwhile the enzyme activity in untreated animals having diabetes was reduced by 24.9% in relation to the standard (24.45±1.50) ?kat/l (р<0.01). The activity data of glutathione peroxidase and glutathione reductase in the retina of white rats having streptozotocin-induced diabetes 2 months after the injection of lipoic acid and quercetin are specified in Table 2 and Diagram (Fig. 2).
When applying lipoic acid on the background of diabetes progress, the activity of glutathione peroxidase rose in the retina to (395.19±22.47) ?kat/g that was equal to 165.0% as compared with the ‘diabetes’ group (239.53±18.27) ?kat/g (р<0.001). Under the quercetin effect, the activity of glutathione peroxidase rose in relation to the untreated animals group by 60.6%, being equal to (384.71±20.80) ?kat/g (р<0.001). Meanwhile the enzyme activity in untreated animals having diabetes was reduced to 45.7% in relation to the standard (524.13±36.45) ?kat/g (р<0.001). So the activity of glutathione reductase in the retina of rats with experimental diabetes progress (2 months) and application of lipoic acid rose to (32.69±1.70) ?kat/g, being equal to 126.7% in relation to the group of diabetic untreated animals (25.81±1.44) ?kat/g (р<0.01), and, when applying quercetin, the activity of glutathione reductase rose by 20.4%, being equal to (31.08±1.67) ?kat/g as compared with the “Diabetes” group (р<0.05). When the diabetes was untreated, the activity of the studied enzyme reduced to 68.2% as compared with the standard (37.84±2.27) ?kat/g (р<0.001). The activity data of glutathione peroxidase and glutathione reductase in blood of white rats having streptozotocin-induced diabetes 6 months after applying lipoic acid and quercetin are specified in Table 3 and Diagram (Fig. 1). In 6 months of diabetes progress and lipoic acid application, the activity of glutathione peroxidase in blood plasma rose to (330.05±23.13) ?kat/g, i.e. as compared with the “Diabetes” group (215.77±18.50) ?kat/g rose by 53.0% (р<0.001). When quercetin was applied, the activity of glutathione peroxidase rose to 45.7% that was (314.37±22.64) ?kat/l in relation to the group of untreated animals (р<0.01). Meanwhile the enzyme activity in untreated animals with diabetes was reduced by 52.3% in relation to the standard (412.56±30.47) ?kat/g (р<0.001). Therefore, the activity of glutathione reductase in blood plasma with the experimental diabetes and application of lipoic acid rose to (20.07±1.22) ?kat/l, i.e. by 20.4% in relation to the group of diabetic untreated animals (16.67±1.04) ?kat/l (р<0.05), and, when applying quercetin, the enzyme activity rose by 17.3% as compared with the “Diabetes” animals group, being equal to (19.56±1.20) ?kat/l. Thereby the studied enzyme activity of diabetic animals was reduced to 68.2% as compared with the standard (24.45±1.50) ?kat/l.
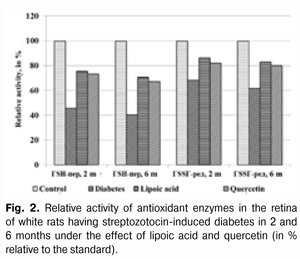
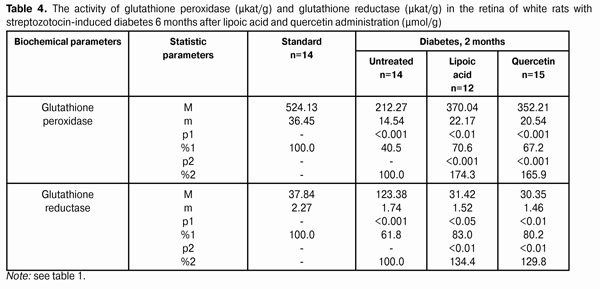
The activity changes of glutathione peroxidase and glutathione reductase in the retina of white rats with streptozotocin-induced diabetes after 6 months of lipoic acid and quercetin administration are specified in Table 4 and Diagram (Fig. 2). In the course of diabetes progress and lipoic acid application, the activity of glutathione peroxidase in the retina of diabetic animals rose to (370.04±22.17) ?kat/g, or 174.3% as compared with the “Diabetes” group (212.27±14.54) ?kat/g (р<0.001).
When quercetin was applied, the activity of glutathione peroxidase rose by 65.9% in relation to the group of untreated animals being equal to (352.21±20.54) ?kat/g (р<0.001). Meanwhile the enzyme activity in untreated animals with diabetes was reduced to 40.5% as compared with the standard (524.13±36.45) ?kat/g (р<0.001). The activity parameter of glutathione reductase in the retina during the diabetes progress and lipoic acid application rose to (31.42±1.52) ?kat/g, i.e. was equal to 134.4% in relation to the group of diabetic untreated animals (23.38±1.74) ?kat/g (р<0.01), and, when applying quercetin, it rose by 29.8% being equal to (30.35±1.46) ?kat/g, as compared with the “Diabetes” group (р<0.01). The enzyme activity with untreated diabetes was reduced to 61.8% as compared with the standard (37.84±2.27) ?kat/g. Generalizing the study results of the antioxidant system state in the retina and blood plasma during the experimental diabetes, it is possible to specify the following. The activity of glutathione peroxidase in blood plasma in the group of untreated animals with diabetes (2 months) was reduced to 39.8%, and in 6 months – by 47.7% in relation to the standard. The activity of glutathione reductase in blood plasma in 2 months was reduced by 24.9%, and in 6 months – by 31.8%. Under the lipoic acid effect, the activity of glutathione peroxidase in blood plasma after two-month diabetes progress rose by 41.1%, and after then – by 53.0% as compared with the untreated animals. Meanwhile the activity of glutathione reductase in blood plasma in 2 and 6 months rose by 20.4% in relation to the untreated animals. After two months of observation, under the quercetin effect, the activity of glutathione peroxidase in blood plasma rose by 36.2%, and after six months – by 45.7%, and the one of glutathione reductase – by 14.9 and 17.3% correspondingly. The activity of glutathione peroxidase in the retina of animals with untreated diabetes (2 months) was reduced by 54.3%, and in 6 months – by 59.5% in relation to the standard while the activity of glutathione reductase was reduced correspondingly by 31.8% and by 38.2% as compared with the standard. Under the lipoic acid effect, the activity of glutathione peroxidase in the retina of white rats after two-month of diabetes progress rose by 65.0%, and after 6 months – by 74.3% as compared with the untreated animals, thereby the activity of glutathione reductase in the retina of animals in 2 months rose by 26.7%, and in 6 months by 34.4% in relation to the group of untreated animals. After the application of quercetin, the activity of glutathione peroxidase in the retina in 2 months rose by 60.6%, and after six months – by 65.9%, the one of glutathione reductase – by 20.4 and 29.8% correspondingly. Our findings show that the application of thiol preparation and bioflavanoid considerably contributes to the stabilization of hydroperoxide dewatering processes in the retina in the conditions of experimental diabetes, while the effect of thiol preparation – lipoic acid occurred to be more expressed as compared with quercetin. Conclusions 1. The application of lipoic acid and quercetin preparations during experimental diabetes (2 and 6 months) considerably allows preventing the inhibition of antioxidant system enzymes in the retina of animals. It is confirmed by the rise in glutathione peroxidase activity in the retina by 65.0% (in 2 months) and by 74.3% (in 6 months) when lipoate is applied, and the rise of glutathione reductase activity by 26.7% (in 2 months) and by 34.4% (in 6 months) in relation to the group of untreated animals. The quercetin application (in 2 months) increases the activity of glutathione peroxidase by 60.6% and after six months – by 65.9%, and the activity of glutathione reductase – by 20.4 and 29.8% correspondingly. 2. Lipoic acid is characterized by the most expressed effect related to the damage degree reduction of the enzymes of the antioxidant system. References 1. Gogina IF, Andriu LV, Ogranovich OE. [Diabetic angio-, retino-, neuropathy: patogenesis, clinic, treatment], Lviv: Liga press; 2000. 186 p. In Ukrainian. 2. Evgrafov VYu. [Diabetic retinopathy: patogenesis, diagnosis, treatment: syn. of thes. ... of M.D.]. М. 1996; 47 p. In Russian. 3. Efimov А, Skorobonskaya N, Zueva N. [Diabetic neuropathy]. Liky Ukrainy; 2005; 3: 21-25. In Russian. 4. Kravchuk EA. [The role of free radical oxidation in pathogenesis of eye diseases]. Vestnik oftalmologii. 2004; 5: 48-51. In Russian. 5. Kryzhanovskaya TV. [Nosotropic rehabilitation aspects of diabetic retinopathy patients]. Mater. of 2-nd Inter. Conf. “Modern Aspects of Cardioendocrinopathy of Ocular Organ”. K. 2005: 73-74. In Russian. 6. Leus NF. [Metabolic development mechanisms and pharmacotherapy aspects of diabetic retinopathy]. Oftalmol Zh. 2003; 5: 75-80. Russian. 7. Nasledov A. [SPSS computer data analysis in psychology and social sciences]. Spb.: Peter; 2005. 416 p. In Russian. 8. Nedzvetskaya OV. [Modern trends in diabetic retinopathy treatment]. Intern. Med. J., 2000; 3: 56-58. In Russian. 9. Olejnik ТV. [The effect of vitamin-coenzyme complexes on antioxidant enzymes retina system during experimental diabetes]. Oftalmol Zh.. 2007; 6.: 58-61. In Russian. 10. Pavlyuchenko KP, Mogilevsky SYu, Chujko AL. [The state of antioxidant enzymes system in the retina during experimental diabetes and application of vatamin В6]. J. Oftalmol Zh. 2011; 3: 73-78. In Russian. 11. Pasechnikova NV, Naumenko VA, Zborovskaya AV. [Blood-retinal barrier state during diabetic retinopathy as per fluorometry data]. Oftalmol Zh. 2008; 5: 4-7. In Russian. 12. Pasechnikova NV. [Laser treatment during ocular fundus pathology]. Pasechnikova NV. Kyiv: Research and production association “Naukova dumka” publishing house of NAS of Ukraine”; 2007. 201 p. In Russian. 13. Sidorova MV. [Diabetic retinopathy. Pathogenesis, clinic, treatment]. K.: SMP “AVERS”; 2006. 156 p. In Russian. 14. Aiello LP, Cahill MT, Wong JS. Systemic considerations in the management of diabetic retinopathy. Am. J. Ophthalmol. 2001; 32: 760-776. 15. Barber A. A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog. In Neuro-Psychopharm. Biol. Psych. 2003; 27: 283 – 290. 16. Bergmeyer HU. Methoden der enzymatischen Analyse. Berlin. 1986; 2220 p. 17. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001; 414: 813-820. 18. Fong DS, Aiello LP, Ferris FL, Klein RK. Diabetic retinopathy. Ophthalmol. 2004; 27: 2540-2553. 19. Jandric-Balen M, Bozikov V, Bistrovic D. Antioxidant enzymes activity in patients with peripheral vascular disease, with and without presence of diabetes mellitus. Coll. Antropol. 2003; 27: 735-743. 20. Halliwell B. The role of oxygen radicals in human disease with particular reference to the vascular system. Haemostasis. 1993; 23: 118-126. 21. Lang GE: Pharmacological treatment of diabetic retinopathy. Ophthalmologica. 2007; 221: 112-117. 22. Muchova J, Liptakova A, Orzaghova Z. Antioxidant systems in polymorphonuclear leucocytes of Type 2 diabetes mellitus. Diabet. Med. 1999; 16: 74-78. 23. Speicher MA, Danis RP, Criswell M. Pharmacologic therapy for diabetic retinopathy. Expert Opion Emerg Drugs. 2003; 8 (1): 239-250. 24. Ulusu NN, Sahilli M, Avci A: Pentose phosphate pathway, glutathione-dependent enzymes and antioxidant argans: effects of stobadine and vitamin E. Neurochem. Res. 2003; 28 (6): 815 – 823. 25. Winkler R, Moser M: Alterations of antioxidant tissue defense enzymes and related metabolic parameters in streptozotocin-diabetic rats-effects of iodine treatment. Wien Klin. Wochenschr. 1992; 104: 409-413
|