J.ophthalmol.(Ukraine).2015;5:35-41.
|
https://doi.org/10.31288/oftalmolzh201553541 Effect of viable cryopreserved human amniotic membrane sutured to cornea on course of experimental bacterial keratitis K.V. Sereda 1, G.I. Drozhzhina 1, T.B. Gaidamaka 1, V.V. Vit 1, V.A. Shabliy 2, G. S. Lobyntseva 2 1 Filatov Institute of Eye Diseases and Tissue Therapy, 2 Institute of Cell Therapy Odessa, Kiev (Ukraine) E-mail: cornea@te.net.ua Background: Since the human amniotic membrane (AM) has the features suggesting the potential for its use in the treatment of bacterial keratitis, and the efficacy of amniotic membrane transplantation for the disease is still poorly understood, the subject matter discussed in the paper is deemed to be relevant. Purpose: To investigate the anti-inflammatory effect of a viable cryopreserved human amniotic membrane (secured using various surgical techniques) in an in vivo model simulating bacterial keratitis. Materials and Methods: In 30 Chinchilla rabbits (30 eyes; weight, 2.5–3.0 kg), the transplantation of a viable amniotic membrane was performed for the previously developed model of moderate bacterial keratitis. The single layer graft inlay was used, and the amnion was secured to the cornea using eight interrupted 10-0 nylon sutures. Animals were followed up for one month. The control group included ten rabbits with experimental bacterial keratitis which received conventional medical therapy. Results: At day 30 after AMT, no lysis and partial lysis of the AM was found in 6/10 eyes and 4/10 eyes, respectively. No stromal edema, diffuse stromal edema, and mild stromal edema were found in 2 eyes, 2 eyes and 6 eyes, respectively. Spotty infiltration and the presence of neovascularization were revealed in 2 eyes and 4 eyes, respectively. Additionally, histology revealed complete epithelialization of the wound surface; however, lamellar differentiation of corneal epithelial cells was observed only in the central cornea. In some eyes, initial stromal neovascularization (especially in the superficial stromal lamellae and around the sutures) was noted. Conclusion: Since in bacteria-induced corneal damage of the same depth the biological covering technique results in a smaller amount of trauma and is accompanied by a lower inflammatory corneal response than those following the AM inlay grafting, the former technique should be preferred to the latter under these circumstances. Key words: bacterial keratitis, cryopreserved amniotic membrane, experiment
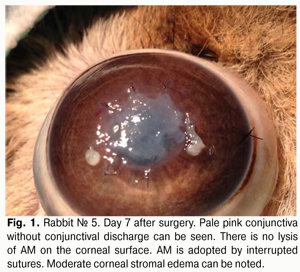
Introduction The potent antibacterial, antiangiogenic, anti-inflammatory and antifibroblastic efficacy of the amniotic membrane (AM) has made it an essential element of surgical techniques for ocular surface reconstruction [1-4] and suggests the potential for its use in the treatment of infectious keratitis. Although the efficacy of AM transplantation for a variety of inflammatory, dystrophic and degenerative disorders of the cornea has been proved in numerous foreign experimental and clinical studies [5-10], its efficacy for bacterial keratitis is still poorly understood [11-13]. Currently, both native and preserved amniotic membranes are used in ophthalmology. The Institute of Cell Therapy (Kyiv) has developed a new AM cryopreservation technique which sustains viability of epithelial and stromal cells of the AM [14]. The technique consists in four-step slow freezing with controlled ice crystal formation for membrane cryopreservation at -196° С with 5% dimethyl sulfoxide cryoprotectant. This cryopreservation technology provides a high level of post-thaw viability of AM cells, thus minimizing a loss of properties of the AM and can facilitate the efficacy of the technique. There is no universal technique for placing the amnion on the wound surface for amniotic membrane transplantation (AMT). There have been numerous contradictory reports concerning the right way to place the AM on the ocular surface. The amnion can be sutured to the ocular surface with its epithelial basement membrane side up and the stromal side down (preferred technique) or stromal side up. The stromal side is sticky, similar to vitreous whereas the epithelium basement membrane side is shiny and non-sticky. There are different modalities of AMT: inlay, overlay and sandwich techniques. In the inlay technique, the AM is placed on the affected area of the cornea, trimmed to fit the size of the epithelial defect, and secured to the cornea with multiple sutures. As a result, the AM functions as a basement membrane over which neighboring recipient corneal epithelial cells can migrate. Different variations of the technique have evolved recently. E.g., additionally, a circular surgical blade is used to perform corneal lamellar dissection (1.5-2 mm) from the stromal-and-epithelial defect to the periphery. Amnion graft is placed epithelial side up. The spatula is used to tuck edges of the graft into a split stroma [18-20]. The overlay technique involves the use of AM to cover the entire cornea and perilimbal area. Here the AM functions primarily as a biological contact lens [19]. The sandwich technique is a combination of the two techniques mentioned before, and is usually performed to treat severe damage to the ocular surface, e.g., deep and extensive corneal ulcers [9,21]. The main purpose of covering the graft with the amnion is to protect it and promote epithelialization [9,22]. The purpose of the study was to investigate the anti-inflammatory effect of a viable cryopreserved human amniotic membrane (secured using various techniques) in an in vivo model simulating bacterial keratitis. Materials and Methods A total of 30 Chinchilla rabbits (30 eyes; weight, 2.5–3.0 kg) were used in the study. The animals were maintained at room temperature and fed with standard laboratory diet. Surgical interventions were performed at the vivarium, the Filatov Institute of Eye Diseases & Tissue Therapy, under aseptic and antiseptic conditions. Animal experiments followed guidelines stipulated by the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Specific Studies (Strasbourg, 1986), the First National Bioethics Congress (Kyiv, 2001), and Law of Ukraine No. 3447–IV On the Protection of Animals from Cruelty (Kyiv, 2006). Moderate experimental bacterial keratitis was induced as described previously. Briefly, the rabbit’s cornea was trephined to two thirds of stromal depth. To infect the cornea, one mL of bacterial suspension of Staphylococcus aureus (109 cell/mL) was instilled two times into the conjunctival cavity, followed by 0.1 mL of subconjunctival injection of diprospan [6]. At 2 weeks post-inoculation, each study eye (30 eyes of 30 study animals) was subjected to AMT. To thaw the membrane, a cryovial was removed from the liquid nitrogen Dewar and placed in 37°-40 °C water bath. Corneal erosion, 3-5 mm in diameter, corneal stromal edema and moderate infiltration were revealed at the time of surgical procedure. Corneal erosion, 3-5 mm in diameter, corneal stromal edema and moderate infiltration were revealed at the time of surgical procedure. The single layer graft inlay was used, and the amnion was secured to the cornea using eight interrupted 10-0 nylon sutures. The upper and lower eyelids of the animal’s eye were sewn together using two “U” sutures, leaving a narrow opening to see through nasally. Animals were followed up for one month. Ten rabbits at a time were sequentially withdrawn from the study and euthanized at post-transplantation day 7, 14 and 30. To this end, a 1.0 m3 air injection was administered intravenously via the marginal ear vein; thereafter, corneal tissue was harvested for morphological examination. The control group included 10 rabbits with experimental bacterial keratitis which received conventional medical therapy. The cells of cryopreserved AM were cultured to determine the post-thaw viability of AM. Method to culture cells of amniotic membrane Initially, cryopreserved amniotic membrane was placed in 37°-40 °C water bath until the liquid phase appeared, and then thawed at room temperature. To the AM immersed in dimethyl sulfoxide solution (DMSO) was added dropwise with constant stirring a solution of Hank’s balanced salt solution (HBSS) at 1:10 ratio. Thereafter, the AM was removed from DMSO solution to HBSS and cut into pieces of 3 mm x 3 mm with scissors. It was removed from HBSS and immersed into alpha-MEM culture medium (HyClone) with 10% Fetal Bovine Serum (Hyclone), 1% RPMI 1640 Amino Acids Solution (Sigma), and 50 ?g/mL streptomycin and 100 units/mL penicillin solution (Sigma). Cell culture was performed at 37°C under a humidified atmosphere of 5% CO2. Clinical investigation Observations were performed daily during a month after AMT to examine the state of lid sutures, nature of conjunctival discharge (if any), and whether the AM was seen on corneal surface through the opening in the medial angle of the eye with partially sutured lids. Lid sutures were removed in newly euthanized animals at day 7, 14 and 30, and the fluorescein test was performed to assess the epithelialization of the corneal surface. Morphological examination Enucleated eye globes were fixed in 10% neutral-buffered formalin and embedded in paraffin wax. Deparaffinized globe sections were stained with hematoxylin and eosin, and van Gieson’s stain and examined with a Jenamed 2 light microscope (Carl Zeiss, Oberkochen, Germany). Results At day 7 after AMT, with the lid sutures removed in 10 rabbits (10 eyes), moderate conjunctival hyperemia and absence of conjunctival discharge was observed in 4/10 eyes and 10/10 eyes, respectively. No lysis, complete lysis and partial lysis of the AM was found in 2/10 eyes, 2/10 eyes and 6/10 eyes, respectively. Epithelialization of corneal surface and moderate corneal stromal edema was noted in 8/10 eyes, and apparent stromal edema was noted in 2/10 eyes (Fig. 1).
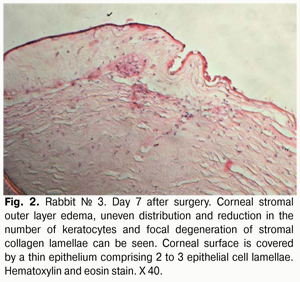
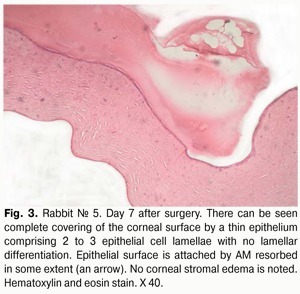
Histology of the corneal tissue revealed stromal edema, with diffuse or focal reduction in the number of keratocytes or uneven distribution of these cells, in 10/10 eyes. The most pronounced edema was observed in superficial corneal stromal lamellae; this was manifested by the development of a reticular stromal structure (Fig. 2). Keratocytes were nearly absent at these sites, and focal mucoid edema of stromal collagen lamellae was revealed in the deeper stroma. No apparent inflammatory alterations were revealed in the central cornea at that observation time point. Inflammatory infiltrates were found near the limbus and around the sutures. As early as day 7 after AMT, the wound was found to be covered with a thin epithelium comprising 2 to 3 epithelial cell lamellae, whereas the AM was found to be resorbed to some extent (Figs. 2,3).
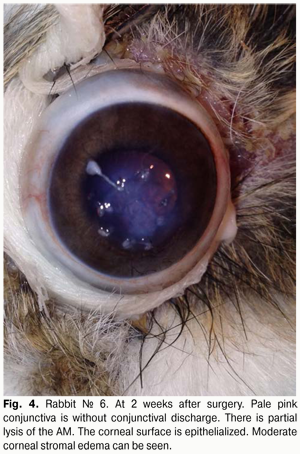
At 2 weeks after AMT, with the lid sutures removed in other 10 rabbits (10 eyes), moderate conjunctival hyperemia and absence of conjunctival discharge was observed in 4/10 eyes and 10/10 eyes, respectively. No lysis and partial lysis of the AM was found in 4/10 eyes and 6/10 eyes, respectively. Corneal surface was re-epithelialized in 10/10 eyes. Moderate corneal stromal edema and vascularization (Fig. 4) was noted in 4/10 eyes and 2/10 eyes, respectively.
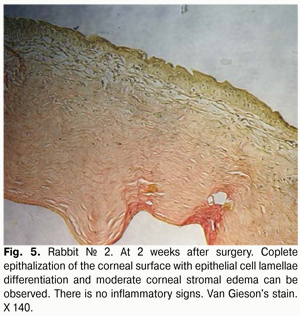
At 2 weeks after surgery, histology revealed corneal stromal edema in fewer eyes, and of lower degree (Fig. 5), compared to a week after surgery. The corneal surface was completely re-covered with the epithelium comprising 5 to 6 epithelial cell lamellae; however, lamellar differentiation of these cells was observed only in the central cornea. It should be noted that such differentiation was absent at the suture sites. In some eyes, epithelialization was completely absent at these sites, and an inflammatory and mostly lymphocytic infiltrate developed around the sutures.
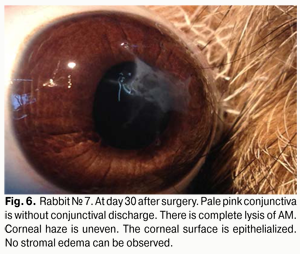
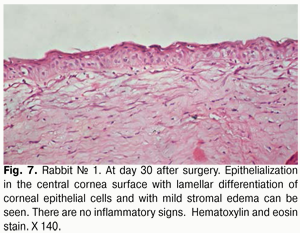
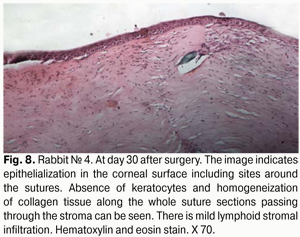
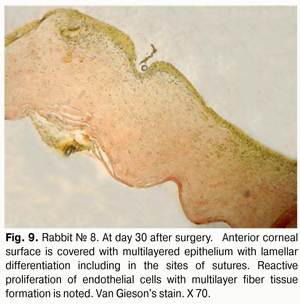
At day 30 after AMT, pink conjunctiva and no conjunctival discharge were observed in 10/10 eyes, and no lysis and partial lysis of the AM was found in 6/10 eyes and 4/10 eyes, respectively. No stromal edema, diffuse stromal edema, and mild stromal edema was found in 2 eyes, 2 eyes and 6 eyes, respectively. Spotty infiltration and the presence of neovascularization (Fig. 6) were revealed in 2 eyes and 4 eyes, respectively. Additionally, complete epithelialization of the wound surface was observed; however, lamellar differentiation of corneal epithelial cells was observed only in the central cornea (Fig. 7). Histology showed thinned epithelium with degenerated epithelial cells and persistent stromal edema around the sutures. It should be noted that degenerated stromal tissue was found along the whole suture sections passing through the stroma (Fig. 8), and reactive proliferation of endothelial cells with the development of multilayered fibrous tissue (Fig. 9) was observed at the inner stroma adjacent to sutures. In some eyes, initial stromal neovascularization (especially in the superficial stromal lamellae and adjacent to sutures) was noted.
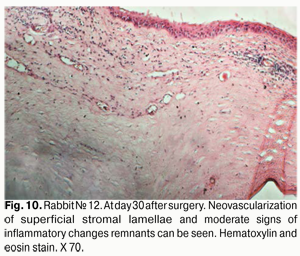
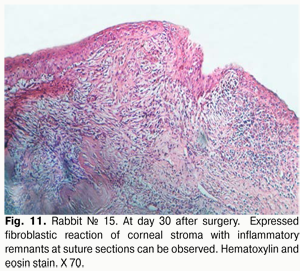
At day 30 after surgery, histology showed that lamellar differentiation of epithelial cells was completed, however, with some sites of thickened epithelium and mild epithelial acanthosis. Insignificant amounts of such cells underwent vacuolar degeneration. This process was accompanied by a reduction in stromal edema. Edema and inflammatory corneal infiltration were persistent at the limbus and sutures. In some eyes, apparent neovascularization and fibrosis of superficial stromal lamellae (Figs. 10, 11) were observed.
Conclusion In the AM inlay grafting involving securing the AM with interrupted sutures to the corneal surface, the inflammatory response was found to be more pronounced compared to that in the biological covering technique [1]. This was evidenced by the presence of moderate central corneal stromal edema and diffuse edema in 18 eyes (60%; 18 animals) and 6 eyes (20%; 6 animals), respectively, by day 30 after surgery. The presence of spotty infiltration in the surrounding recipient cornea was revealed in 6 eyes (20%). A more pronounced inflammatory response observed in the AM inlay grafting compared to that in the biological covering technique may result from the intervention features (securing the AM with interrupted sutures to the corneal surface), and, consequently, an increased amount of trauma to the cornea. Postoperatively, presence of sutures on the corneal surface was accompanied by increased production of proteolytic enzymes and provided mechanical irritation leading to the development of an inflammatory response with neovascularization in 12 eyes (40%; 12 animals) by the end of their observation period. Additionally, a less pronounced anti-inflammatory effect could be caused by a smaller size of the amniotic membrane in the AM inlay grafting compared to that in the biological covering technique. Therefore, since in corneal bacterial infections of the same severity the biological covering technique results in a smaller amount of trauma and is accompanied by a lower inflammatory corneal response than those following the AM inlay grafting, the former technique should be preferred to the latter under these circumstances. References 1.Kjaergaard N, Hein M, Hyttel L et al. Antibacterial properties of human amnion and chorion in vitro. Eur J Obstet Gynecol Reprod Biol. 2001;94:224–229. 2.Talmi YP, Sigler L, Inge E, Finkelstein Y et al. Antibacterial properties of human amniotic membranes. Placenta. 1991;12:285–8. 3.Hao Y, Ma DH, Hwang DG et al. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea. 2000; 19(3): 348-52. 4.Tseng SC, Li DQ, Ma X. Suppression of transforming growth factor-beta isoforms, TGF-beta receptor type II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix. J Cell Physiol. 1999;179:325–35. 5.Solomon A, Meller D, Prabhasawat P et al. Amniotic membrane grafts for nontraumatic corneal perforations, descemetoceles, and deep ulcers. Ophthalmology. 2002;109:694-703. 6.Gris O, Del Campo Z, Wolley-Dod C et al. Amniotic membrane implantation as a therapeutic contact lens for the treatment of epithelial disorders. Cornea. 2002;21:22–7. 7.Khokhar S, Natung T, Sony P, et al. Amniotic membrane transplantation in refractory neurotrophic corneal ulcers: a randomized, controlled clinical trial. Cornea. 2005;24:654–60. 8.Prabhasawat P, Tesavibul N, Komolsuradej W et al. Single and multilayer amniotic membrane transplantation for persistent corneal epithelial defect with and without stromal thinning and perforation. Br J Ophthalmol. 2001 Dec;85(12):1455-63. 9.Seitz B. Amniotic membrane transplantation. An indispensable therapy option for persistent corneal epithelial defects. Ophthalmologe. 2007;104:1075–1079. 10.Thatte S. Amniotic membrane transplantation: an option for ocular surface disorders. Oman J Ophthalmol. 2011;5:67-72. 11.Gicquel JJ, Bejjani RA, Ellies P. Amniotic membrane transplantation in severe bacterial keratitis. Cornea. 2007;26:27–33. 12.Barequet IS, Habot-Wilner Z, Keller N. Effect of Amniotic Membrane Transplantation on the Healing of Bacterial Keratitis. Invest Ophthalmol Vis Sci. 2008; 49(1): 163-7. 13.Kim JS, Kim JC, Hahn TW et al. Amniotic membrane transplantation in infectious corneal ulcer. Cornea. 2001;20:720–6. 14.Lobyntseva GS et al, inventors. [Method for operating cryobank of biological objects]. Ukrainian patent No. 49759. 2004 Nov 15. Ukrainian. 15.Novitskii IIa. [Place of amniotic membrane transplantation in treatment of corneal diseases accompanied by neovascularization]. Vestn Oftalmol. 2003;(6):9-11. Russian. 16.Novitskii IIa, Sarakhman MN, Smal’ TM. [Amniotic membrane transplantation with its securement in corneal layers]. Oftalmokh. 2003;(3):4-7. Russian. 17.Stepanov VK, Ivanov OV. [Use of amniotic membrane as a protective biological bandage of affected cornea and as a corneal graft in keratoplasty]. In: [Proceedings of the 9th Russian Ophthalmological Congress]; Moscow (Russia): 2010. p. 319. Russian. 18.Drozhzhina GI, Sereda EV, Gaidamaka TB et al. [Influence of the corneal biological covering with cryopreserved amniotic membrane on the peculiarities of the course of modeled bacterial keratitis]. Oftalmol Zh. 2015;(1):103-109. Russian. 19.Аzuara-Blanco A, Pillai CT, Dua HS. Amniotic membrane transplantation for ocular surface reconstruction. Br J Ophthalmol. 1999;35:399-402 20.Dravadzhian ZKh, Ambariumian AV, Ovakimian AV [Use of amniotic membrane in corneal perforation]. In: [Proceedings of Russian National Ophthalmological Forum]; 2009 Oct 8-9; Moscow (Russia): Moscow Helmholtz Institute if Eye Diseases; 2009. p. 280-4. Russian. 21.Sippel KC, Ma JJ, Foster CS. Amniotic membrane surgery. Curr Opin Ophthalmol. 2001;12:269-81. 22.Seitz B, Resch M, Schl?tzer-Schrehardt U et al. Histopathology and ultrastructure of human corneas after amniotic membrane transplantation. Arch Ophthalmol. 2006; 124:1487–90. 23.Drozhzhina GI, Vansovich EV, Gaidamaka TB, inventors. [Method for modeling moderate bacterial keratitis]. Ukrainian patent No. 87119. 2014 Jan 27. Ukrainian.
|