J.ophthalmol.(Ukraine).2015;5:25-29.
|
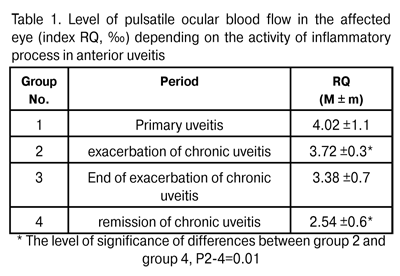
https://doi.org/10.31288/oftalmolzh201552529 Ocular hemodynamics and activity of autonomic nervous system at different stages of the course of anterior uveitis N.I. Khramenko, Cand. Sc. (Med) Filatov Eye Disease and Tissue Therapy Institute, the NAMS of Ukraine, Odessa E-mail: khramenkon@mail.ru Background: Given the complex pathogenetic mechanism of the development of uveitis, it is important to investigate the features of the interaction between the immune, vascular and nervous systems, the level of their activity, and the potential for preventing the development of irreversible morphological changes. Purpose: To investigate the hemodynamic state of the eye and activity of autonomic nervous system in anterior uveitis at different stages of the inflammatory process. Materials and Methods: The study involved 66 anterior uveitis patients. They underwent general clinical examination and ophthalmic rheography involving measurement of pulsatile ocular blood flow (POBF, indicated by RQ, ‰ index), maximum blood flow velocity in the eye (? index, Om/s), and vascular tone (alpha/T percentage index) with Reocom, the computerized rheography apparatus. Results and Discussion: The level of POBF was found to depend on the level of activity of the process, with the Spearman correlation rank rho= 0.26 (P < 0.05). The lowest RQ index values, RQ = (2.54 ± 0.6)‰, were found in steady remission of chronic uveitis, and were statistically significantly lower by 32% (P < 0.05) than those in exacerbation of the disease. Ocular blood flow velocity changes were similar to those of pulsatile ocular blood flow. A direct relationship was revealed between index RQ (‰) and erythrocyte sedimentation rate (ESR, mm/h), an indicator of inflammatory activity (rho = 0.45, P < 0.05). In patients with anterior uveitis complicated by macular edema, the RQ levels and the tone of major ocular vessels during exacerbation episodes were found to be by 54% (P = 0.02) and by 35.5% (P = 0.001), respectively, higher than those in patients with uncomplicated anterior uveitis. In anterior uveitis patients with macular edema, the LF index was by 46% higher (P = 0.02) than in those with uncomplicated anterior uveitis, that is, excessive sympathicotonia was accompanied by retinal damage. Conclusion: We found the ocular hemodynamics features of anterior uveitis depending on the stage of inflammatory process, and the mechanisms of influence of different subsystems of the autonomic nervous system on the hemodynamic state of the eye and complicated course of uveitis. Keywords: anterior uveitis, ocular hemodynamics, autonomic nervous system INTRODUCTION Uveitis is one of the severest ocular inflammatory disorders with a wide range of etiologies and complex pathogenesis. Of importance is that recurrent acute episodes of the disease result in new functional and morphological damage, and the rate of complications resulting in visual incapacitation is rather high. It is well established that immune system may be involved in the uveitic process. In an inflammatory ocular process, the structure and the blood supply of ocular tunics are of special importance. Since the choroid may serve as a depot of immune cells, it functions as a lymph node; additionally, under certain circumstances, it becomes a center of immune response [1]. The issues of neurohumoral regulation of immune response (i.e., interaction of the immune system with the integrating body systems, the nervous and endocrine systems) are of an increasing importance for medical science [2,3]. The autonomic nervous system (ANS) plays an important, if not crucial, role in supporting homeostasis. It uses two functionally different divisions, the sympathetic nervous system (SNS) and the parasympathetic nervous system (PSNS), to regulate the functions of internal organs and metabolism. When the tone of the SNS increases, mobilization of energy substrates occurs, and the body is being prepared to respond to external stimuli, whereas activation of the PSNS stimulates the accumulation of energy resources and intensification of plastic response [2-4]. The development of infection syndrome is known to be associated with increased tone of the SNS, and is accompanied by characteristic alterations in immunity [5,6]. Active involvement of the vascular system in inflammatory process is reflected in increased arterial and venous vascularity, altered tone features and morphology of vessel walls and blood flaw rheology, and is associated with the direct effect of inflammatory mediators (neuropeptides, acetylcholine, histamine, bradykinin, prostaglandins etc.) and modulators (norepinephrine and adrenaline). The level of this involvement is ultimately determined by the functional state of higher regulatory systems (the nervous, endocrine and immune systems). Given the complex pathogenetic mechanism of the development of uveitis, it is important to investigate the features of the interaction between the immune, vascular and nervous systems, the level of their activity, and the potential for preventing the development of irreversible morphological changes. The purpose of the work was to investigate the hemodynamic state of the eye and activity of autonomic nervous system in anterior uveitis at different stages of the inflammatory process. Materials and Methods Sixty six cases of anterior uveitis were treated either as inpatients of Inflammatory Ocular Pathology Department of the Institute or as outpatients. Their ages ranged from 20 to 47 years. Patients with secondary glaucoma were excluded from the study. We used the anatomic classification of uveitis [7] based on the principle that anterior uveitis includes iritis, anterior cyclitis and iridocyclitis. The stage of inflammatory process (i.e., either primary process, exacerbation, post-treatment outcome of inflammation, or remission stage) was taken into account as a clinical index. Patients underwent assessment of the level of visual acuity, biomicroscopy, indirect ophthalmoscopy, ocular tonometry, perimetry, and examination of the electrical sensitivity of the optic nerve and critical frequency of phosphene disappearance. Additionally, they underwent ophthalmic rheography involving measurement of pulsatile ocular blood flow (POBF, indicated by RQ, ‰ index), maximum blood flow velocity in the eye (? index, Om/s), and vascular tone (alpha/T percentage index) with Reocom, the computerized rheography apparatus. In the affected eye, the mean best-corrected visual acuity (BCVA) was 0.4±0.04, and the intraocular pressure (IOP) varied from 18 to 22 (20.4±1.0) mmHg. The analysis of autonomic regulation involved cardiac rhythm variability (CRV) to assess the state of the mechanisms regulating physiological functions of the body, including general activity of regulatory mechanisms, neurohumoral regulation of the heart, and quantitative relationships between the SNS and PSNS. Electrocardiographic (ECG) signal was registered at one of the standard ECG leads (the second ECG lead) while the patient was still lying on his back in bed. The ECG was recorded for at least 5 minutes. The investigation procedure was preceded with a 5-10 min period of adaptation to the new environment. The ECG was registered with the computerized apparatus using the software application to calculate the CRV parameters automatically. We used a number of the most important statistic and spectral indices recommended as international standards by the 1996 Task Force of The European Society of Cardiology and The North American Society of Pacing and Electrophysiology [8]: TP (ms2), total power (of spectral analysis) reflecting the total activity of regulatory mechanisms of the ANS; VLF (%), percentage of power in the very low frequency component of the spectrum, supposed to reflect the central sympathetic energotropic component of the ANS; VLF characterizes the influence of higher autonomic centers on cardiovascular lower centre, and reflects the state of neurohumoral, hormonal and metabolic regulation levels. VLF is used as a marker of the degree of relationship between autonomic and supra-autonomic (including hypothalamic-pituitary and cortical) levels of circulation control; LF (%), the percentage of total power in the low frequency component of the spectrum which characterizes sympathetic activity and the activity of the vasomotor centre; HF (%), the percentage of total power in the high frequency component of the spectrum which corresponds to the level of parasympathetic activity; LF/HF ratio, index of vagal-sympathetic interaction. The statistical analysis involved the use of pairwise Student t test, nonparametric Mann Whitney test, Pearson correlation analysis and nonparametric Spearman correlation analysis. Results and Discussion Rheographic index RQ (‰) was used to determine the level of pulsatile ocular blood flow (POBF). The level of POBF was found to depend on the level of activity of the process, with the Spearman correlation rank rho= 0.26 (P < 0.05). The highest RQ index values were found in patients with primary uveitis (Table 1). However, they were widely variable, thus evidencing a dramatic loss of adaptation of the hemodynamic system at the onset of disease. The lowest RQ index values, RQ = 2.54 ± 0.6 ‰, were found in steady remission of chronic uveitis, and were statistically significantly lower by 32% (P < 0.05) than those in exacerbation of the disease (Table 1).
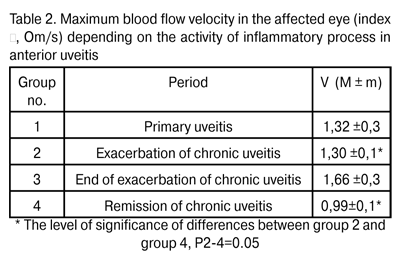
Ocular blood flow velocity changes were similar to those of pulsatile ocular blood flow, with the lowest levels (V=0.99±0.1 Om/s) observed in remission of chronic uveitis, and being statistically significantly lower by 23.8% (V= 1.30 ± 0.1 Om/s, P < 0.05) than those in exacerbation of the disease (Table 2).
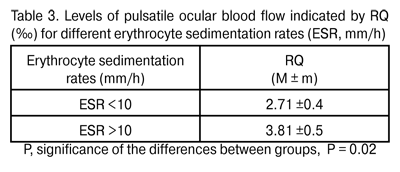
A direct relationship was revealed between index RQ (‰) and erythrocyte sedimentation rate (ESR, mm/h), an indicator of inflammatory activity (rho = 0.45, P < 0.05). When patients were divided into those with low and high ESR values (ESR < 10 mm/h and ESR ? 10 mm/h, respectively), there was a statistically significant difference in pulsatile ocular blood flow (Table 3) between these two groups, with the mean RQ in the latter patients (RQ= 3.81 ± 0.5 ‰) being by 28.9% higher (P = 0.06) than that in the former.
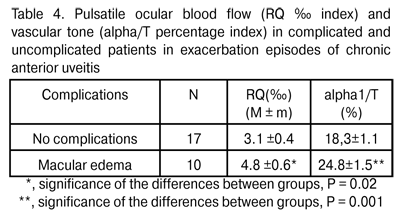
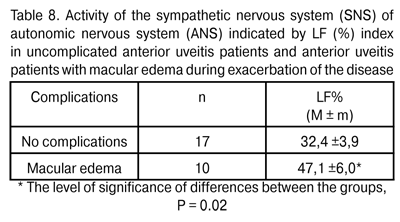
Therefore, the ocular circulatory system responds to the activation of inflammatory process by intensifying the ocular vascular blood flow, which is likely to improve the tissue supply with oxygen, energy complexes, and even with immune system components, thus contributing to sanation. Previously [9], we have revealed the increase in maximum choroidal thickness to 335±32 ?m in exacerbation of anterior uveitis, compared to the norm of 315.5±22.3 ?m (P = 0.02). That is, in different periods of inflammatory process, we have revealed both morphological changes (increase in vascular bed volume) and functional changes in the ocular blood supply system. It should be noted that inflammatory process is a typical and the most common adaptive and protective response of the body, and ideally should be of a limited amount, exerting no damage to organs and tissues. In patients with anterior uveitis complicated by macular edema, the RQ levels and the tone of major ocular vessels during exacerbation episodes were found to be by 54% (P = 0.02) and by 35.5% (P = 0.001), respectively, higher than those in patients with uncomplicated anterior uveitis (Table 4). That is to say, during exacerbation episodes of chronic anterior uveitis, the vascular response in the former patients was found to be more aggressive than that in the latter.
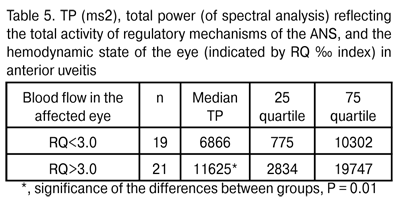
The onset, development, course and outcome of inflammation depend on the reactivity of the body, which is determined in its turn primarily by the functional state of higher regulatory systems (the nervous, endocrine and immune systems). The autonomic nervous system exerts considerable influence on the development of inflammation. Effectors of the nervous, endocrine and immune systems (neuromediators, neuropeptides, hormones and lymphokines) exert a direct regulating influence on tissue, vessels, blood, hemo- and lymphopoiesis. Moreover, these effectors exert the influence mediated by other inflammatory mediators, with the release of the latter being modulated by them through specific receptors of cell membranes and changes in the intracellular levels of cyclic nucleotides. The regulating influence of the neuroendocrine system on the immune system and on the production of pro- and anti-inflammatory cytokines has been reported [2,3,10]. Given the integrity of the body, continuous interaction of these systems through neurotransmitters and cytokines [2,3], association of immune response parameters with higher nervous activity and the activity of ANS subsystems, the alterations in either system can either compensate for or exacerbate alterations in another one, thus determining the clinical features of the course of disease. The analysis of the influence exerted by the ANS, its centres and periphery, on the ocular blood supply system in anterior uveitis has been performed. The uveal system (choroid, ciliary body and iris) is known to be involved in sympathetic and parasympathetic regulation [11, 12]. A direct relationship was revealed between the total activity of central and peripheral regulatory mechanisms of the ANS (indicated by TP) and POBF (indicated by RQ, ‰ index) (rho = 0.38, P < 0.05). Thus, an increase in ocular blood flow values was accompanied by an increase in TP values (Table 5).
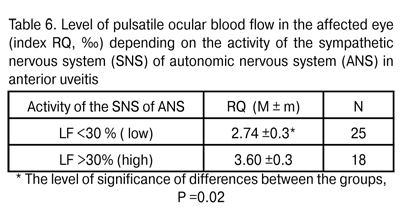
According to Baevskii [13], increased TP values mean activation of the lower control levels (here, it means local regulation). Therefore, activation of blood flow is accompanied by that of more autonomic (that is, those being closer to the target organ) subsystems of the ANS. Total activity (i.e., power) of the ANS consists of the activities of humoral, sympathetic and parasympathetic arms. A more detailed analysis revealed that in patients with anterior uveitis, increased influence of the sympathetic arm of the ANS (to be specific, activation of the vasomotor centre indicated by LF index value) led to increased regional blood flow. A direct relationship was revealed RQ (‰) index and LF (rho = 0.32, P < 0.05). When patients were divided into those with low (LF<30%) and high (LF>30%) activity of the sympathetic arm, there was a statistically significant (by 23.8%) increase in pulsatile ocular blood flow indicated by RQ ‰ index (Table 6).
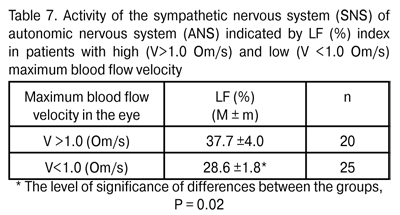
Additionally, a relationship was revealed between the activity of the SNS of ANS and maximum blood flow velocity in the eye (? index, Om/s). In patients with low blood flow velocity (V <1.0 Om/s) in the eye, the percentage of SNS activity in regulating mechanisms was 28.6±1.8%, compared to a greater percentage of SNS activity, 37.7±4.0% (P = 0.02), in those with higher blood flow velocity (V>1.0 Om/s).
Therefore, increased pulsatile ocular blood flow and maximum blood flow velocity in the affected eye during activation of the inflammatory process are associated with activation of the sympathetic arm of autonomic nervous system. Within the process of activation of regional blood flow, the influence of sympathetic arm (the latter being closer to target organ and more mobile than humoral system) is increased at the expense of humoral influence of the higher command level, the hypothalamic-pituitary system. Thus, a direct relationship was revealed between the increase in LF/VLF ratio with predominance of sympathetic over humoral regulation and the increase in index RQ (‰) (rho = 0.42, P < 0.05). It is a positive sign, since the prevalence of humoral regulation would strengthen the already existing prolonged hyperreactive stress with either stable or intensified alteration-and-damage phases [14]. Conclusion 1. In anterior uveitis, the ocular circulatory system responds to the activation of inflammatory process by intensifying the blood flow, which is reflected in the increase in pulsatile ocular blood flow and ocular blood flow velocity by 32% and 23.8%, respectively, in the presence of increased influence of the sympathetic arm of the autonomic nervous system. 2. In anterior uveitis complicated by macular edema, the activity of sympathetic arm of the autonomic nervous system was found to be by 46% higher than in uncomplicated anterior uveitis. 3. We found the ocular hemodynamics features of anterior uveitis depending on the stage of inflammatory process, and the mechanisms of influence of different subsystems of the autonomic nervous system on the hemodynamic state of the eye and complicated course of uveitis. References
1. Avetisov SE, Egorov ЕА, Moshetova LК, Neroyev VV, Takhchidi HP. [Ophthalmology: National Guideline]. Moscow: GEOTAR-Media;2008. 944 p. Russian
2. Abramov VV. [Integration of immune and nervous systems]. Novosibirsk: Nauka;1991. 168 p. Russian
3. Abramov VV. [Possible principles of integration of immune and neuroendocrine systems]. Immunologiia. 1996;1:60-61. Russian
4. Vein AM. [Autonomic abnormalities: clinical picture, diagnosis and treatment. A Manual for Physicians]. Moscow: MIA;2000. 752 p. Russian
5. Raginene IG. [Dependence of functional status of immune system on initial autonomic tone of the body]. [Abstract of Cand. Sc. thesis]. Tomsk (Russia): State Siberian Medical University; 2003. 23 p. Russian
6. Tracey KJ. The inflammatory reflex. Nature. 2002 Dec 19-26;420(6917):853-9.
Crossref 7. Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of Uveitis Nomanclature (SUN) Working Group. Am J Ophthalmol. 140:509-516.
8. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of The European Society of Cardiology and The North American Society of Pacing and Electrophysiology. Eur Heart J. 1996 Mar;17(3):354-81.
9. Khramenko NI, Konovalova NV, Shaibi Abderrakhim. [Investigation of sensory retina and choroid of uveitis patients with optical coherence tomography]. Vostok-Zapad. Tochka zreniia. 2014;1:200-3. Russian
10. Sorokin OV, Markova EV, Trufakin SV. [New aspects of involvement of autonomic nervous system in immune regulation]. Neiroimmunologiia. 2013;2(1):136-7. Russian
11. Lutjen-Drecoll E. Choroidal innervation in primate eyes. Exp Eye Res. 2006 Mar;82(3):357-61.
Crossref 12. May CA, Lutjen-Drecoll E. Choroidal ganglion cell changes in human glaucomatous eyes. J Glaucoma. 2004; 13: 389–95.
Crossref 13. Baevsky RM. [Analysis of heart rate variability: history and philosophy, theory and practice]. Clin Informat and Telemed. 2004;1:54-64. Russian
14. Iabluchanskii NI, Martynenko AV, Isaieva AS. [Basics of application of non-invasive examination of human regulatory system]. Kharkiv: Osnova; 2000. p.26 Russian
|