J.ophthalmol.(Ukraine).2015;5:18-24.
|
https://doi.org/10.31288/oftalmolzh201551824 Two-year results of treatment of open-angle glaucoma and concomitant cataract with combined phacoemulsification and Alcon EX-PRESS glaucoma filtration implant surgery S. P. Medvedchuk,1 PhD candidate, P.A. Bezdetko,2 Dr. Sc. (Med), Prof., G.Ia. Parkhomenko,2 Cand. Sc. (Med) 1 "New Vision" Clinic Group, Cherkasy, Kyiv, Khmelnytsky, Dnipropetrovsk, Sumy, Ukraine, 2 Department of Ophthalmology, Kharkiv National Medical University E-mail: ck-maindoctor@zir.org.ua Background: A combined approach for glaucoma coexistent with cataract has been always interesting for ophthalmologists since these diseases are often found together in patients. Purpose: To investigate the clinical efficacy and safety of combined phacoemulsification and Alcon EX-PRESS glaucoma filtration device (GFD) implant procedure and to develop indications for implantation of 50-?m and 200-?m devices. Materials and Methods: The study involved 115 primary open-angle glaucoma patients (115 eyes) with cataract divided into 3 groups depending on the type of glaucoma surgery. Group 1 involved 30 patients (30 eyes) who underwent phacoemulsification combined with the 50-?m GFD implant procedure. Group 2 involved 30 patients (30 eyes) who underwent phacoemulsification combined with the 200-?m GFD implant procedure. Group 3 included 55 patients (55 eyes) who underwent phacoemulsification combined with nonperforating deep sclerectomy. The mean preoperative intraocular pressure (IOP) in Group 1, Group 2 and Group 3 was 22.9±2.9 mmHg, 22.8 ± 3.5 mmHg (р1/2 > 0.05), and 22.6 ± 3.4 mmHg (р1/3 > 0.05; р2/3 > 0.05), respectively. Results: At 24 months postoperative, the mean IOP in Group 1, Group 2 and Group 3 was 15.4 ± 1.7 mmHg, 16.1 ± 2.1 mmHg (р1/2 > 0.,05), and 18.8 ± 1.9 mmHg (р1/3 < 0.05; р2/3 < 0.05), respectively. The number of postoperative complications in Group 1 and Group 2 was statistically significantly lower than that in Group 3. Conclusion: Phacoemulsification combined with the EX-PRESS glaucoma filtration device implant procedure is an efficacious and safe surgical technique for stabilization of glaucoma process. The 50-?m Alcon EX-PRES GFD implantation is indicated in stage I or stage II of unstabilized glaucoma with subcompensated IOP. The 200-?m Alcon EX-PRESS GFD implantation is indicated in stage III or stage IV of unstabilized glaucoma with decompensated IOP. Keywords: open-angle glaucoma, EX-PRESS glaucoma filtration device, intraocular pressure, cataract phacoemulsification.
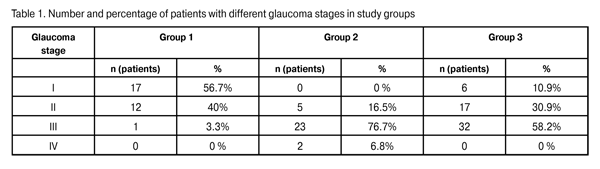
Introduction Whether a combined approach is preferred over sequentially staged surgeries for primary open-angle glaucoma (POAG) coexistent with cataract is still a matter of controversy among ophthalmologists. It is possible to identify a number of the most debatable questions in the treatment of a primary glaucoma patient with cataract. First, whether to do a combined procedure or a two-stage surgery? Second, which intervention should be performed first in a two-stage surgery? Third, if the IOP becomes normalized after cataract extraction, whether the glaucoma surgery is always necessary [1-3]? Early after cataract extraction, the IOP decreases as a result of decreased aqueous production and simultaneously increased aqueous outflow. It is associated with a widening of the anterior chamber angle and improvement of trabecular filtration [4-7]. Thereupon, however, the IOP increases, and some 30% of patients with advanced glaucoma and 50% of those with far-advanced glaucoma require further treatment for the disease [1-3, 8, 9]. A miniature glaucoma drainage device developed by Belkin and Glovinsky for filtration of intraocular fluid has been used since 1998. Later on, Alcon began manufacturing the device under the EX-PRESS name. The advantages of the of the EX-PRESS glaucoma drainage device implantation include the simplicity and microinvasive nature of the intervention, the possibility of using a uniform procedure and performing it in the outpatient setting, fewer complications after surgery, etc. [10, 11]. The importance of the study is determined by its purpose and objectives as follows: to investigate the clinical efficacy and safety of surgical treatment of POAG and cataract using the Alcon EX-PRESS glaucoma filtration device (GFD) implantation combined with phacoemulsification, and to develop indications for implantation of dia. 50-?m and 200-?m dia. devices. Material and Methods The study involved 115 POAG patients (51 men and 64 women) with cataract (115 eyes). Patient age varied from 56 to 75 (range, 69.2±9.2) years. Patients were divided into three groups depending on the type of glaucoma surgery. Group 1 involved 30 patients (30 eyes) who underwent phacoemulsification combined with the 50-?m GFD implant procedure. Group 2 involved 30 patients (30 eyes) who underwent phacoemulsification combined with the 200-?m GFD implant procedure.Group 3 included 55 patients (55 eyes) who underwent phacoemulsification combined with nonperforating deep sclerectomy (NPDS). Patients underwent assessment of the level of visual acuity, ocular pneumatic tonometry with Topcon (Japan), electronic tonography with GlauTest-60 (Russia), ophthalmic biomicroscopy with Nidek slit lamp, Goldmann lens gonioscopy and pachymetry. Additionally, patients underwent ganglion cell complex (GCC) and optic nerve head (ONH) thickness measurements with the RTVue-100 optic coherent tomography (OCT). In Group 1, 96.7% of eyes were diagnosed with stage I glaucoma or stage II glaucoma and 3.3% of eyes were diagnosed with stage III glaucoma. Seventy per cent of Group 1 patients had IOP > 21 mmHg with a mean ± SD number of glaucoma medications of 2.3 ± 0.4. These facts provided the basis for implantation of the 50-?m dia. EX-PRESS GFD in the eyes of Group 1. In Group 2, 16.5% of eyes were diagnosed with stage II glaucoma and 83.5% of eyes were diagnosed with stage III glaucoma or stage IV glaucoma. Seventy six point seven per cent of Group 2 patients had IOP > 21 mmHg with the mean number of glaucoma medications of 2.5 ± 0.5. These facts provided the basis for implantation of the 200-?m dia. EX-PRESS GFD in the eyes of Group 2. In Group 3, 10.9%, 30.9% and 58.2% of eyes were diagnosed with stage I glaucoma, stage II glaucoma and stage III glaucoma, respectively. Operations were performed by one surgeon using the same protocol. The Infinity® Vision System (Alcon) was used for phacoemulsification. Technique for the phacoemulsification combined with 200-?m dia. EX-PRESS GFD implantation A mixture of 0.2 mL of mitomycin C (MMC) 0.02% (0.2 mg in 1 mL) with 0.2 mL of marcain solution (5 mg in 1 mL), with an exposure time of 4 minutes, was injected subconjunctivally. The MMC solution was drained from under the conjunctiva using appropriate tools. The conjunctiva was separated from the cornea at the limbus, and diathermal coagulation of the episcleral vessels was performed. A 1/2 thickness rectangular scleral flap (4x4 mm) was fashioned and dissected forward until clear cornea. Three 1.0-mm corneal paracenteses were made at 2, 7, and 11 o’clock, and 0.1 mL of Phenylephrine hydrochloride 0.1% with 0.1 mL of marcain solution was instilled into the anterior chamber through the paracentesis. Viscoelastic (Viscoat, Supreme) was then injected into the anterior chamber. A corneal tunnel was created with a 2.2-mm blade at the 9 o’clock position. A 5-5.5-mm continuous circular capsulorhexis was performed with gripping forceps. Hydrodissection and hydrodelineation of the nucleus was carried out. The choice of nucleus phacoemulsification modality depended on the density of the lens nucleus. Bimanual irrigation-aspiration of lens material was performed using an irrigation-aspiration system. Viscoelastics (Provisc + Supremе) were introduced in the capsular bag. The IOL was implanted in the capsular bag using a cartridge and injector. Viscoelastic (Supremе) was introduced into the anterior chamber. The anterior chamber was penetrated, and paracentesis was created with a 25-gauge needle in the blue/grey zone of the limbus parallel to the iris plane. The 200-?m dia. EX-PRESS device was introduced through the paracentesis into the anterior chamber and positioned parallel to the iris plane. The scleral flap was secured with 4 interrupted sutures. The conjunctiva was then closed with 2 interrupted sutures. Viscoelastic was aspirated using the irrigation-aspiration system. Corneal incisions were hydrated with balanced salt solution.
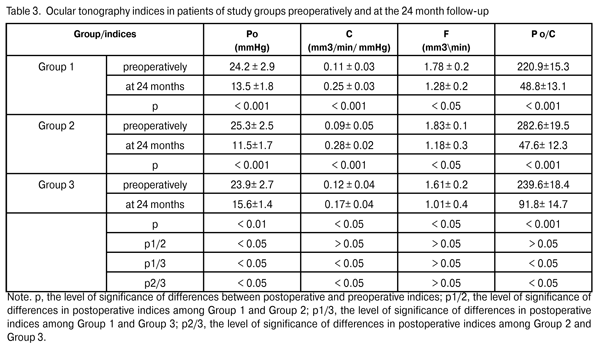
Preoperatively, all patients used topical hypotensive medications (prostaglandin analogs, alone or in combination with beta blockers; carbonic anhydrase inhibitors; alpha-2 agonists; and combinations of above medications). The mean preoperative IOP in Group 1 was 22.9±2.9 mmHg, with an IOP of up to 21 mmHg in 30% of the eyes, and above 21 mmHg in 70% of the eyes. The mean preoperative IOP in Group 2 was 22.8±3.5 mmHg (p1/2 > 0.05), with an IOP of up to 21 mmHg in 23.3% of the eyes, and above 21 mmHg in 76.7% of the eyes. The mean preoperative IOP in Group 3 was 22.6±3.4 mmHg (p1/3 > 0.05; p2/3 > 0.05), with an IOP of up to 21 mmHg in 20% of the eyes, and above 21 mmHg in 80% of the eyes (Table 2). Thirty per cent of patients (9 eyes) of Group 1, 23.3% of patients (7 eyes) of Group 2 and 20% of patients (11 eyes) of Group 3, were diagnosed with unstabilized glaucoma at a maximal regimen of hypotensive medications (prostaglandin analogs in combination with beta blockers; carbonic anhydrase inhibitors; alpha-2 agonists) and an IOP of up to 21 mmHg, and therefore underwent glaucoma surgery. The clinical efficacy and safety of phacoemulsification combined with the EX-PRESS GFD implantation were assessed based on the preoperative and postoperative number of hypotensive medications, dynamics of changes in the GCC and NFL thickness, improvement in visual acuity, and number of intra- and postoperative complications. We compared preoperative indices and those at the 24-month follow-up. Statistical analysis was performed using Wilcoxon signed-rank test (Statistica 6.0). Results and Discussion The ocular hypotensive surgery effect of surgery was reflected in a significant reduction in IOP levels and significant improvement in ocular hydrodynamics. At the 12-month postoperative follow-up, the mean IOP level in Group 1, Group 2, and Group 3 decreased by 27 % to 16.7 ± 1.2 mmHg (р < 0.001), 28.9% to 16.2 ± 1.8 mmHg (р < 0.001; р1/2 > 0.05), and 25.7% to 16.8 ± 2.2 (р < 0.01; р1/3 > 0.05; р2/3 > 0.05), respectively. At the 24-month postoperative follow-up, the mean IOP level in Group 1, Group 2, and Group 3 decreased by 32.7% to 15.4±1.7 mmHg (р < 0.001), 29.4% to (16.1 ± 2.1) mmHg (р < 0.001; р1/2 > 0.05), and 16.8% to 18.8 ± 1.9 mmHg (р < 0.05; р1/3 < 0.05; р2/3 < 0.05), respectively. We found a statistically significant reduction in IOP levels in all study groups, and an insignificant difference in indices among the groups at 12-month postoperative. At 24 months postoperative, the hypotensive effect in Group1 and Group 2 was maintained at 29.4% to 32.7% levels, whereas that in Group 3 was found to be statistically significantly reduced to 16.8%. Moreover, a statistically significant reduction in all basic ocular tonography indices was found in all study groups at 24 months postoperative (Table 3).
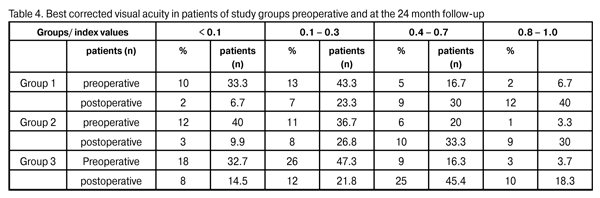
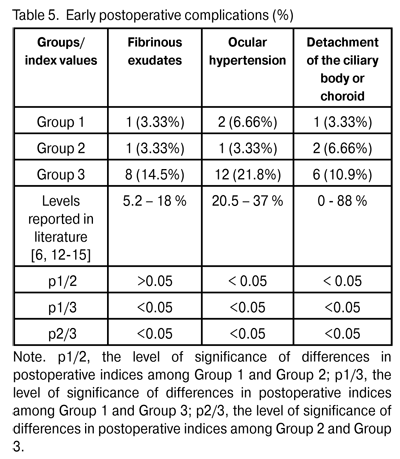
At 12 months and 24 months postoperative, the number of hypotensive medications used to achieve a target IOP in Group 1 reduced from 2.3±0.4 to 0.57±0.05 (p < 0.05) and to 0.47±0.03 (p < 0.05), respectively, whereas that used to achieve a target IOP in Group 2 reduced from 2.5±0.5 to 0.57±0.05 (p < 0.05) and 0.44±0.06 (p < 0.05; р1/2 > 0.05), respectively. At 12 months and 24 months postoperative, the number of hypotensive medications used to achieve a target IOP in Group 3 reduced from 2.6±0.5 to 0.49±0.02 (р1/3 > 0.05; р2/3 > 0.05) and 0.75 ± 0.06 (p < 0.05; р1/3 < 0.05; р2/3 < 0.05), respectively. We found a statistically significant reduction in the number of hypotensive medications in all study groups, and an insignificant difference in these indices among the groups at the 12-month postoperative follow-up. Additionally, the number of hypotensive medications used to achieve a target IOP in Group 3 was statistically significantly larger than those in Group 1 and Group 2, and the difference in these indices among the groups was insignificant at the 24-month postoperative follow-up. Visual acuity is the basic parameter needed for the detection and management of any ocular disorder. In glaucoma, the reduction in visual acuity is not an early sign of the disease; however, vision is worsened significantly and may be lost in an advanced and final glaucoma stage, respectively. In all the study cases, visual acuity improved postoperatively (Table 4). The level of visual improvement depended on the stage of glaucoma and presence of concomitant retinal disorders. No intraoperative complications were observed in Groups 1 and 2, whereas 11 cases (20%) of trabecular microperforation were observed in Group 3. Early postoperative complications occurred within first three postoperative days and included fibrinous exudates, ocular hypertension, and detachment of the ciliary body or choroid (Table 5).
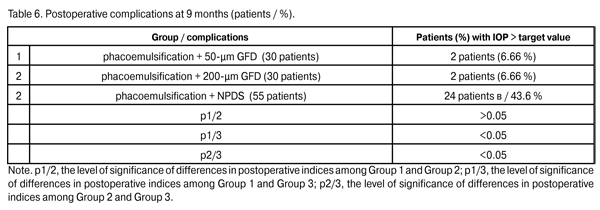
Fibrinous exudates were found in Group 1, Group 2, and Group 3, in 1 eye (3.3%), 1 eye (3.3%), and 8 eyes (14.5%), respectively (р1/3 < 0.05, р2/3 < 0.05). Ocular hypertension was found in Group 1, Group 2, and Group 3, in 2 eyes (6.6%), 1 eye (3.3%), and 12 eyes (21.8%), respectively (р1/3 < 0.05, р2/3 < 0.05). Detachment of the ciliary body or choroid was found in Group 1, Group 2, and Group 3, in 1 eye (3.3%), 2 eyes (6.6%), and 6 eyes (10.9%), respectively (р1/3 < 0.05, р2/3 < 0.05). Patients with fibrinous exudates received a comprehensive anti-inflammatory treatment. Ocular hypertension was compensated with medical treatment (beta blockers and carbonic anhydrase inhibitors). In all cases with detachment of the ciliary body or choroid, reattachment was observed in the presence of conservative treatment. Therefore, the rates of intraoperative and early postoperative complications in Group 1 and Group 2 were found to be lower than those in Group 3 and those reported in literature. Any surgery for glaucoma can be deemed successful if the target IOP level is achieved at the late follow-up. Cicatrization of surgical wounds created for aqueous outflow is still the main problem of glaucoma surgery [9, 15]. A marked proliferative process in the area of surgical intervention results in a recurrent increase in IOP followed by further decompensation of glaucoma process (late postoperative complications) [4,13,14,16-18]. Although an increase in the number of patients with the IOP > target value was observed in Group 1 and Group 2 at 9 months postoperative (Table 6), the percentage of these patients (6.6%) in each of this groups was statistically significantly less than that in Group 3 (43.6%; р1/3 < 0.05, р2/3 < 0.05).
Topical hypotensive medications (including prostaglandin analogs and beta blockers) were administered to all patients with increased IOP. In 1 patient (3.3%) of Group 1 and 1 patient (3.3%) of Group 2, a revision of a filtering bleb for the IOP decompensation was performed using our technique. In 1 patient (3.3%) of Group 1 and 1 patient (3.3%) of Group 2, the increase in IOP was compensated using topical hypotensive medications. Laser goniopuncture to the Descemet's window was performed in 30 eyes (54.5%) of Group 3 at 9 months postoperative.
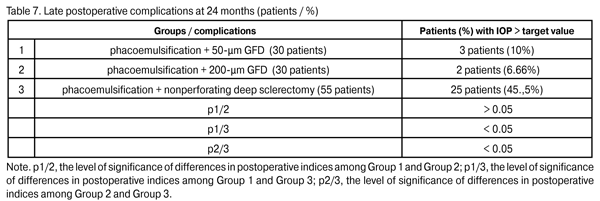
In 5 patients (9.1%) of Group 3, a repeat glaucoma surgery (implantation of the 200-?m GFD) for the IOP decompensation was performed and resulted in the IOP compensation. In the rest 19 Group 3 patients (34.5%) with the IOP decompensation, it was managed with topical hypotensive medications. At 24 months, the percentage of patients with IOP > target value in Group 2 (6.66%) did not change, whereas that in Group 1 decreased to 10%; however, these percentages were statistically significantly lower than that in Group 3 (p1/3 < 0.05, p2/3 < 0.05). Topical hypotensive medications (including prostaglandin analogs and beta blockers) were administered to all patients with increased IOP and contributed to the achievement of the target IOP.
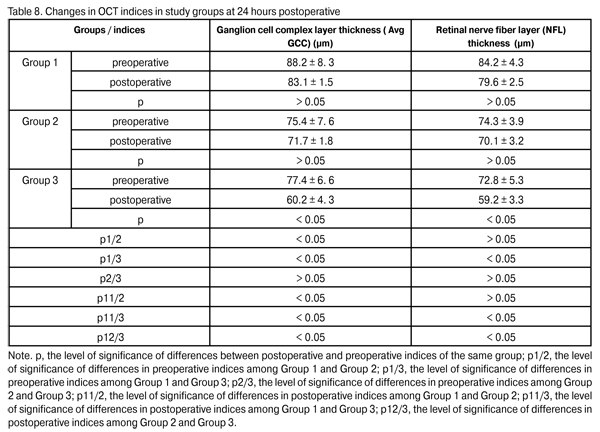
It has been established and verified by morphological studies that ganglion cell death and correlation of GCC thickness with that of retinal nerve fiber layer (NFL) of the optic nerve are the phenomena that the progress of pathological process in POAG is based on [ 9, 19-21]. Preoperative, a statistically significant difference in average GCC layer thickness (Avg GCC) and mean NFL thickness was found only among Group 2 and group 3 patients (p2/3, > 0.05). At 24 hours postoperative, Avg GCC in Group 1, Group 2 and Group 3 was 83.1 ± 1.5 ?m, 71.7 ± 1.8 ?m (p1/2 < 0.05), 60.2 ± 4.3 ?m (p1/3 < 0.05; p2/3 < 0.05), respectively. At 24 hours postoperative, NFL thickness in Group 1, Group 2 and Group 3 was 79.6 ± 2.5 ?m, 70.1 ± 3.2 ?m (p1/2 > 0.05), 59.2 ± 3.3 ?m (p1/3 < 0.05; p2/3 < 0.05), respectively (Table 8).
These results demonstrated a statistically insignificant (p > 0.05) change in GCC layer and NFL thickness in Group 1 and Group 2 at 24 hours postoperative, thus evidencing stabilization of glaucoma process. A statistically significant change in these indices was found in Group 3, thus evidencing progress in glaucoma. Conclusion 1. Phacoemulsification combined with the EX-PRESS glaucoma filtration device implant procedure is an efficacious surgical treatment for open-angle glaucoma concomitant with cataract. At 24 months postoperative, the IOP was compensated in 90% of cases of Group 1 (phacoemulsification combined with 50-?m GFD implantation), 93.4% of cases of Group 2 (phacoemulsification combined with 200-?m GFD implantation), and 53.4% of cases of Group 3 (phacoemulsification combined with nonperforating deep sclerectomy), with a statistically significant decrease in the number of hypotensive medications in all groups. A statistically insignificant (p > 0.05) change in GCC layer and NFL thickness in Group 1 and Group 2 at 24 hours postoperative evidenced stabilization of glaucoma process. 2. The absence of intraoperative complications and a statistically significantly lower rate of early postoperative complications in Group 1 and Group 2 than that in Group 3 suggest that phacoemulsification combined with the Alcon EX-PRESS glaucoma filtration device implantation is a rather safe procedure. 3. The 50-?m Alcon EX-PRES GFD implantation, with the number of topical hypotensive medications of more than 2, is indicated mostly in stage I or stage II of unstabilized glaucoma with subcompensated IOP. 4. The 200-?m Alcon EX-PRESS GFD implantation at a maximal possible number of topical hypotensive medications is indicated mostly in stage III or stage IV of unstabilized glaucoma with uncompensated or subcompensated IOP at a maximal possible number of topical hypotensive medications, or any stage of glaucoma process with uncompensated IOP at a maximal regimen of topical hypotensive medications.
References
1. Zbitneva SV. [Optimization of phacoemulsification treatment combined with non-perforating deep sclerectomy for cataract and glaucoma], [Abstract of Cand. Sc. (Med) thesis]. Kyiv: Shupik National Medical Academy of Continuing Education; 2008. Russian
2. Mogilevskii SIu. [Cataract with concomitant primary glaucoma (pathogenetically oriented surgery and laser treatment)]. [Abstract of Dr. Sc. (Med) thesis]. Donetsk: Gor’kii Donetsk Medical University; 2007. Russian
3. Pavliuchenko KP, Mogilevskii SIu, Oleinik TV. [Efficacy of phacoemulsification, intraocular lens implantation and trabeculectomy in cataract patients with primary glaucoma]. Oftalmol Zh. 2005;2:13-7. Russian
4. Borras O, Echaque J. Study: non-perforating “Shlemmectomy” an effective glaucoma treatment option. Ocular Surg News. 2000;18(18): 24-5
5. Ceki? O, Batman C. Hyposecretion of aqueous: another mechanism for reduced intraocular pressure after phacoemulsification [letter; comment]. J Cataract Refract Surg. 1998 May;24(5):574
Crossref 6. Lachkar Y, Neverauskiene J, Jeanteur-Lunel MN et al. Nonpenetrating deep sclerectomy: a 6-year retrospective study. Eur J Ophthalmol. 2004 Jan-Feb;14(1):26-36
Crossref 7. Meyer MA, Savitt ML, Kopitas E. The effect of phacoemulsification on aqueous outflow facility. Ophthalmology. 1997 Aug;104(8):1221-7
Crossref 8. Neroev VV, Bykov VP, Kvasha OI, Belyovtseva TA. [Glaucoma micro drainage surgery: literature review]. Klin. oftalmologiia 2009;3:113-6. Russian
9. Nesterov AP. Glaucoma. Moscow: Meditsina; 1995.
10. Kuroedov AV, Ogorodnikova VIu. [Late results of use of EX-PRESS glaucoma drainage device in patients with advanced stages of primary open-angle glaucoma]. Oftalmologiia 2012;1:38-42. Russian
11. de Jong L, Lafuma A, Aguad? AS, et al. Five-year extension of a clinical trial comparing the EX-PRESS glaucoma filtration device and trabeculectomy in primary open-angle glaucoma. Clin Ophthalmol. 2011;5:527-33
Crossref 12. Alekseev BN, Pisetskaia SF. [Ocular hydro- and hemodynamics in detachment of the ciliary body or choroid following glaucoma surgery]. Oftalmol Zh. 1983;6:347-49. Russian
13. Chung AN, Aung T, Wang JC, Chew PT. Surgical outcomes of combined phacoemulsification and glaucoma drainage implant surgery for Asian patients with refractory glaucoma with cataract. Am J Ophthalmol. 2004 Feb;137(2):294-300
Crossref 14. Jurowski P, Go? R. Effectiveness of the combined surgical treatment for glaucoma and cataract. Klin Oczna. 2005;107(4-6):212-6
15. Ku WC, Lin YH, Chuang LH, Yang KJ. Choroidal detachment after filtering surgery. Chang Gung Med J. 2005 Mar;28(3):151-8
16. Blumenthal M. Manual ECCE, the present state of the art. Klin Monbl Augenheilkd. 1994 Nov;205(5):266-70
Crossref 17. Bloomberg L.B. Modified trabeculectomy / trabeculotomy with no-stitch cataract surgery. J Cataract Refract Surg. 1996 Jan-Feb;22(1):14-22
Crossref 18. Munden PM, Alward WL. Combined phacoemulsification, posterior chamber intraocular lens implantation, and trabeculectomy with mitomycin C. Am J Ophthalmol. 1995 Jan;119(1):20-9
Crossref 19. Zueva MV, Sheludchenko VM, Shpak AA. [Up-to-date ocular diagnostic techniques: summary and prospects]. In: [Proceedings of the 9th Russian Ophthalmological Congress]; Moscow (Russia): 2010. p. 498-500. Russian
20. Egorov EA, Kurmangalieva MM, Fefotovskikh GV. [Morphological changes in the retina of glaucoma patients]. Klin. oftalmologiia 2004;5(2):54-6. Russian
21. Mosin IM. [Optical coherent tomography]. In: Shamshinova AM, ed. [Essays on clinical ocular physiology]. 3rd ed. Moscow: Nauchno-meditsinskaia firma MBN; 2006. p.785-658
|