J.ophthalmol.(Ukraine).2015;5:7-12.
|
https://doi.org/10.31288/oftalmolzh20155712 Diagnosis and Treatment of Acanthamoeba Keratitis Lincoln Lavado Landeo Medicine at San Marcos National University (Lima) ABSTRACT Objective: Review the clinical characteristics, diagnosis, treatment and visual results in patients diagnosed with Acanthamoeba keratitis (AK). Demonstrate the importance of early diagnosis and of prompt and effective treatment. Material and methods: Retrospective study of 14 eyes in the same number of patients diagnosed with AK, treated at Centro Visi?n between July 2008 and June 2012. All the cases were confirmed by smear and/or culture. Two groups were established: early and late diagnosis. Treatment was carried out with propamidine and polyhexamethylene biguanide. After the infection had been eliminated, final visual acuity and duration of treatment were recorded. Results: The most frequently affected group was aged between 21 and 40 years (9 cases). Only two eyes (14.3%) were correctly diagnosed initially as AK. Eleven patients (78.6%) were contact lens wearers. The most common sign was diffuse infiltrate (62.3%); perineural infiltrate was only seen in one case. Five patients were diagnosed within the first thirty days (early diagnosis group) and nine cases were diagnosed later (late diagnosis group). Median visual acuity in the early diagnosis group was 20/40, and in the late diagnosis group it was 20/400. The median duration of treatment in the early diagnosis group was six months, and in the late diagnosis group it was ten months. Conclusions: The majority of the eyes (85.7%) were initially erroneously classified as keratitis resulting from other causes. When AK is diagnosed early there is a better visual prognosis, and also prolonged treatment will not be necessary. Key words: Acanthamoeba; keratitis; contact lenses; polyhexamethylene biguanide; propamidine. INTRODUCTION Acanthamoeba is an opportunistic, free-living non-parasitic amoeba which lives in soil, all types of water (fresh water and saltwater) (1,2), and in saliva. For this reason, there is an elevated potential for infection. This high exposure is confirmed by the fact that more than 80% of immunocompetent individuals possess serum antibodies against Acanthamoeba (3). A total of 24 species have been identified, but only certain genotypes are pathogens. The precise mechanism for corneal infection is not known. It is believed that it is related to several factors, including epithelial trauma, a large inoculum of organisms and defense mechanisms of the host. Acanthamoeba keratitis (AK) was first described in the United States in 1973, in a post corneal trauma case (4). The link between the use of contact lenses (CL) and AK was described in 1984. At first the main risk factor described was the use of CL in environments containing contaminated water. Subsequently, it was found that keratitis was also associated with the use of homemade diluted saline solution used to sterilize contact lenses. All types of CL have been linked to AK (5), but it is the prolonged use of daily wear soft contact lenses (SCL) which represents the greatest risk (6, 7). It is now known that this infection can also occur in non-contact lens wearers (8, 9). AK has been identified in practically every part of the world (10-15). It begins through contact with contaminated water, both in healthy and immunocompromised individuals. It can also occur after corneal injury involving vegetable matter (16). MATERIAL AND METHODS All the patients diagnosed with AK and examined by Centro Visi?n between July 2008 and June 2012 were included in this study. Clinical and microbiological histories were reviewed retrospectively. The following variables were taken into consideration: age, gender, predisposing factors, duration of symptoms, prior diagnosis and treatment, ocular findings at the time of examination, treatment and final vision obtained. In all patients a microbiological study was made which was consistent in the following terms: Gram, Giemsa and PAS staining; blood agar cultures; Sabouraud and non-nutrient E. coli enriched agar. All organisms, including Acanthamoeba, were identified using standard procedures. Those patients with less than one month since the onset of symptoms were classified as early diagnosis cases, while the rest, with more than 30 days, were classified as late diagnosis cases. Once diagnosis had been made through microbiological study, treatment was begun with 0.2% polyhexamethylene biguanide (PHMB) and 0.1% propamidine isethionate. In order to compare the post-treatment visual acuity and duration of treatment of the two groups –early and late diagnosis- the non-parametric Wilcoxon rank-sum test was applied. In both cases, the referential significance level was ? = 0.05. The statistical treatment of the data was carried out using the STATA 12.0 version package. RESULTS Over a period of four years, fourteen eyes were treated, belonging to the same number of patients. There were an equal number of male and female patients (7:7). The average age was 24.9 years, ranging from 14 to 40 years. Young adults aged between 21 and 40 were the most frequently affected, with a total of nine cases found in this group (64.3%). Table 1 shows the distribution of patients according to age and gender. 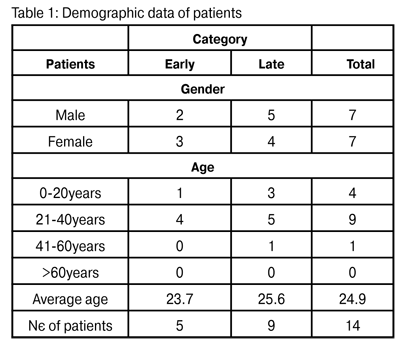 At the time of the first examination, twelve patients (85.7%) were incorrectly classified as bacterial keratitis. Nine cases were treated as bacterial keratitis (64.3%), two cases were treated as viral keratitis (14.3%) and one case received antifungal treatment. One of these patients even received topical corticosteroids. Only two of the eyes (14.3%) were diagnosed correctly as AK.
Eleven patients (78.6%) were SCL users, and four of these reported that they had not followed the correct cleaning and replacement protocols. Three patients (21.4%) were not CL users, but reported the following related circumstances: vegetable origin trauma, fall of a drop of milk from a dairy produce storage tank, and bathing in water stored in a cistern.
In all cases the condition was unilateral. The patients displayed the following symptoms: photophobia, tearing, ocular pain –in some cases very intense- and decreased visual acuity. The most significant symptoms, detected using a slit lamp examination, are described in Table 2.
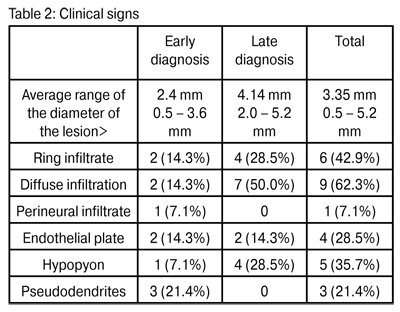 Diffuse infiltration was the most frequent symptom (62.3%). Ring infiltration, albeit partial in some cases, was found in six eyes (42.9%). Hypopyon was observed in five cases (35.7%). Pseudodendritic keratitis, which is often confused with herpetic keratitis, was seen in three eyes (21.4%). Finally, perineural infiltrate, sometimes considered the result of pathognomic amoeba infection (17), was only observed in one case (7.1%).
In all cases diagnosis was based on demonstration in the laboratory of Acanthamoeba cysts or trophozoites in the smear and/or positive culture of the samples obtained through a corneal scrape, following topical anesthesia. In four cases, (two early diagnoses and two late diagnoses) it grew in a form associated with S. epidermidis.
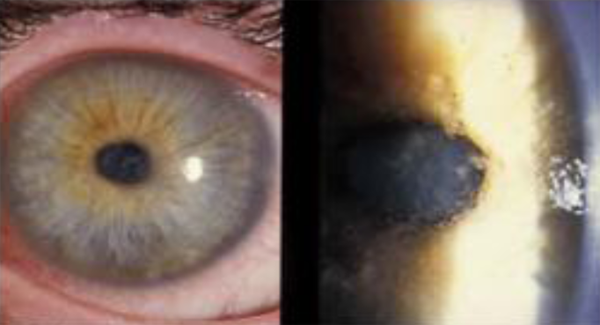
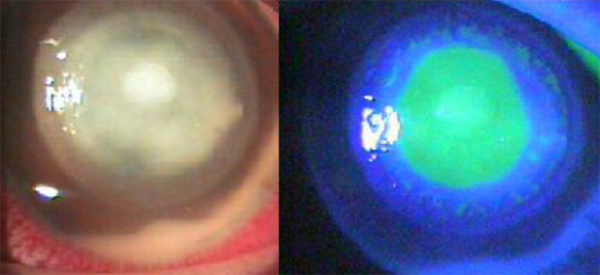
Five patients were diagnosed within the first thirty days of evolution and were classified as early diagnosis patients (Fig. 1). Nine patients, with more than thirty days of illness, were classified as late diagnosis patients (Fig. 2).
Figure 1:Patient with Acanthamoeba keratitis in an early epithelial stateFigure 1:Patient with Acanthamoeba keratitis in an early epithelial state
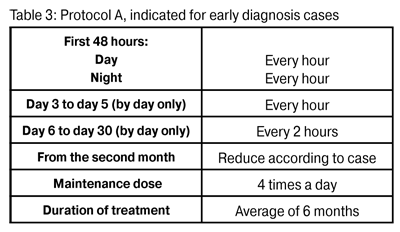
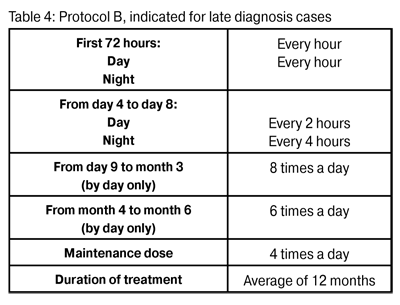
Figure 2: Patient with Acanthamoeba keratitis in a late stateFigure 2: Patient with Acanthamoeba keratitis in a late state Treatment was begun as soon as the presence of amoeba was confirmed. The frequency of treatment was related to the duration of infection. In early diagnosis cases Protocol A was used (Table 3). In late diagnosis cases Protocol B was used (Table 4).
The application of these protocols was flexible, given that both groups continued to receive treatment until they were considered cured. In twelve of the fourteen patients, the infiltration was resolved with the treatment described. In two late diagnosis cases, because of the seriousness of the symptoms, the condition was treated with a conjunctival flap, with the flap maintained for a period of between six and eight weeks.
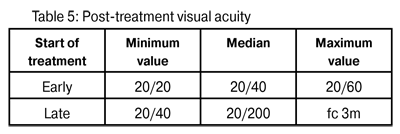
In cases where associated bacterial infections were found, 0.3% gatifloxacin eye drops were used. In all the eyes 1% topical atropine was employed. In none were topical corticosteroids indicated. In the majority of patients a conditional oral analgesic was recommended. In early diagnosis cases pre-treatment visual acuity fluctuated between 20/40 and 20/100; in late diagnosis cases between 20/60 and a 1 m finger count (fc). The median post-treatment visual acuity of the first group was 20/40 and in the second 20/200 (p = 0.0125) (Table 5.).
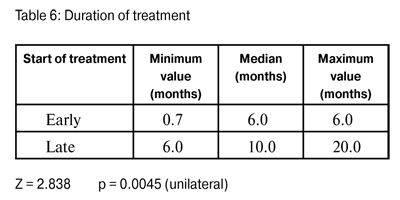
An evaluation was also made of the duration of treatment necessary for achieving elimination of the microorganism. In cases where treatment was initiated early, the median was six months, and when treatment began late the median was ten months (p=0.0045) (Table 6).
DISCUSSION Acanthamoeba is a protozoan that resists a number of environmental conditions. In unfavorable conditions it changes its phenotype and transforms itself into a cyst (18). The cystic form is resistant to a number of agents, which represents a serious problem when it comes to treatment. When conditions become favorable again, the cysts return to their infective trophozoite form, leading to renewed tissue infection. Acanthamoeba keratitis should be considered in cases of corneal trauma associated with contaminated soil or water, and in all contact lens users. This diagnosis should also be taken into consideration when the onset is slow, and if there is a failure in the response to first-line therapy for bacterial keratitis or herpes simplex, including when there has been a positive culture for these organisms. In this study S. epidermidis co-infection was found in four cases (28.6%). The majority of infected patients are young adults. We observed that 64.3% of cases occurred in patients aged between 21 and 40 years, regardless of the gender of the patient. The majority of cases (85.7%) had been classified erroneously as keratitis due to other causes. This led to a delay in appropriate treatment. That is why it is important to be familiar with the clinical characteristics of AK and be open at all times to an alternative diagnosis (19). Nor is it certain that this infection is always associated with the use of contact lenses (20), given that 21.4% of cases occurred in other associated circumstances. All our patients had a unilateral condition. Uncomplicated AK presents a common progressive epithelial to stromal pattern. The majority of patients complain of pain, photophobia and tearing. In early AK, the pain is usually severe and disproportionate to the clinical signs, but the absence of pain does not preclude diagnosis (21). In this study, across all patients, the most frequent sign was diffuse infiltrate (62.3%), followed by ring infiltrate (42.9%) and hypopyon (35.7%). The clinical characteristics of AK typically vary in accordance with the duration of the condition. In the early stages punctate keratopathy, pseudodendrites, epithelial or sub-epithelial infiltrations and perineural infiltrate, with ring infiltrate, may be observed. In this research pseudodendrites were observed in 21.4% of cases, and perineural infiltrate in just one patient (7.1%). This type of infiltration occurs as a consequence of the clustering of trophozoites around the nerves, and it is considered practically pathognomonic. In this stage anterior uveitis is uncommon. The more advanced condition is characterized by ring infiltrate, ring-like ulceration, secondary sterile anterior uveitis and hypopyon (Fig. 3). Sometimes a disciform reaction or the presence of endothelial plates may lead to corneal edema. Hypoesthesia and pericorneal infiltrates may also occur. In our study, half the eyes exhibited diffuse edema, and 28.5% ring infiltrate or hypopyon. In this stage, progression is more common to more severe forms, such as scleritis, the formation of abscesses, cataracts, corneal melting and/or perforation, and posterior segment inflammation. The definitive diagnosis in all cases was made based on a smear and/or culture. In our context we do not have access to amoeba DNA sequencing through polymerase chain reaction assay (PCR). Treatment was begun as soon as the presence of the amoeba was confirmed. Frequency of treatment was related to the duration of infection. In early cases, Protocol A was used, and in late cases Protocol B was used, although application of these was flexible. In all cases treatment was continued until it was established that the condition had been cured. To date, there are no approved licensed drugs for AK in any country. Trophozoites are susceptible to many available chemotherapeutic agents, such as for example: antibiotic, antiseptic, antifungal, antiprotozoal, antiviral and antineoplastic agents. But persistent infection is associated with the presence of cysts, and very few drugs are effective against these. Only cysticidal substances are considered effective in anti-amoebic treatment.
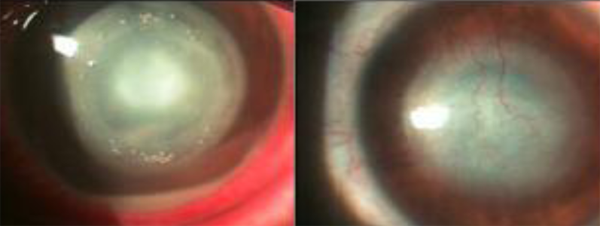
Figure 3: 14 year-old female, contact lens using patient with advanced Acanthamoeba keratitis. Ring infiltrate and mild hypopyon can be observed on the left. On the right, the same eye is shown after treatment, where a central leucoma scar with some blood vesselscan be seen.
Currently, diamidines and biguanides are the most effective in vitro cysticidal drugs, and their use is supported by a significant series of cases (22, 23). The biguanide used in this study was 0.02% (200?g/ml) polyhexamethylene biguanide (PHMB). Concentrations higher than 0.02% have been used by other authors in resistant cases (24). Biguanides interact with the cytoplasmic membrane, producing a loss of cellular components and inhibition of respiratory enzymes. In vitro studies have shown that PHMB –in common with chlorhexidine- offers greater amoebicidal and cysticidal activity than the other drugs studied. Biguanides are first-line drugs in treatment for this keratitis, and an addictive or synergistic effect is believed to exist with diamidines (25).
The available diamidines are propamidine isethionate and hexamidine. The drug used in our patients was 0.1% propamidine. The cationic properties of diamidines induce structural changes in the membrane which affect cellular permeability. When they penetrate the amoebic cytoplasm, a denaturation of the cytoplasmic proteins and enzymes is produced. While it is effective against trophozoites and Acanthamoeba cysts, propamidine should not be used as a single treatment for this condition (26). Diamidine is well tolerated by ocular tissues, although over a prolonged period it may lead to toxic keratopathy.
In all the eyes topical 1% atropine was used. In cases associated with bacterial infections, 0.3% gatifloxacin topical eye drops were indicated.
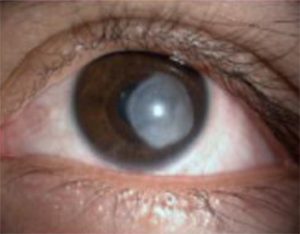 Figure 4: 28 year-old, male, contact lens using patient, with advanced Acanthamoeba keratitis. A thick leucoma can be seen occupying almost the entire post-treatment central cornea
Post-treatment median visual acuity was better in those cases treated early (20/40) than in those cases treated late (20/400) (Fig. 4), representing a significant statistical difference (p=0.0125). The median duration of treatment in early diagnosed cases was six months, and in late diagnosed cases duration was ten months. This difference is also statistically significant (p=0.0045). All of the above demonstrates that AK represents a considerable challenge in terms of clinical treatment. If it is diagnosed early, in addition to the potential for better visual recovery, a shorter treatment period will also be necessary. These findings are consistent with those found by Dartet al (26).For that reason, in order to ensure a good result, a rapid early diagnosis is essential. If treatment is delayed for more than three or four weeks the prognosis worsens.
A link has been proposed recently between riboflavin and ultraviolet radiation (cross-linking) in the treatment of AK, given the potent tissue oxidizing action (27-29), which represents a promising option to anti-infection medical treatment in cases of infectious refractory corneal disease (30,31).
The prevention of AK is the best treatment. Contact lens using patients should be carefully educated in the proper use and care of contact lenses. Use of contact lenses in swimming pools, jacuzzis or at the beach is not recommended. Contamination of rigid contact lenses with saliva should be avoided. In addition, this condition should be treated by a professional with experience in dealing with such cases.
BIBLIOGRAPHY
1.Ram?rez Flores E, Robles Valderrama E, Sainz Morales MG y col. Coliformes y amibas de vida libre presentes en agua subterr?nea. RevistaLatinoamericana de RecursosNaturales 2009; 5(2): 98-105. 2.Todero Wink MR, Caumo K, BrittesRott M. Prevalence of Acanthamoeba from Tap Water in Rio Grande doSul, Brazil. CurrMicrobiol 2011; 63: 464-469. 3.Abbot RL and Elander TR. Acanthamoeba Keratitis. En: Tasman W, Jaeger EA. Duane Clinical Ophthalmology, Philadelphia: JB Lippincott, 1995; 4: 18. 4.Illingworth CD, Cook SD. Acanthamoeba Keratitis. SurvOphthalmol 1988; 42(6): 493-508. 5.Kerr NM, Ormonde S. Acanthamoeba keratitis associated with cosmetic contact lens wear. The New Zealand Medical Journal 2008; 121 (1286). 6.Legarreta JE, Nau AC, Dhaliwal DK. Acanthamoeba keratitis associated with tap water use during contact lens cleaning: manufacturer guidelines need to change. Eye Contact Lens. 2013; 39:158-161. 7.Donzis PB, Mondino BJ, WeissmanA et al. Microbial analysis of contact lens care systems contaminated with Acanthamoeba. Am J Ophthalmol 1989; 108: 53-56. 8.Dua HS, Azuara-Blanco A, Hossain M et al. Non-Acanthamoeba amebic Keratitis. Cornea 1988; 17(6): 675-677. 9.Tu EY. Acanthamoeba keratitis: A new normal. Am J of Ophthalmology 2014; 158(3): 417-419. 10.Aasly K, Bergh K. Acanthamoeba keratitis; report of the first Norwegian cases. ActaOphthalmologica 1992; 70(5): 698-701. 11.Carrette S, Marechal-Courtois CH, Hernandez J et al. A propos d'un cas de k?ratite ? Acanthamoeba Bull. Soc. BelgeOphtalmol 2000; 275: 49-53. 12.Fathallah A, Rayana NB, Knani L et al. Les k?ratites ? Acanthamoeba sp. A propos de 3 cas diagnostiqu?s au centre tunisien. La Tunisie M?dicale 2010; 88(2): 111-115. 13.Walochnik J, Haller-Schober EM, Kolli H et al. Acanthamoeba-Keratitis in ?sterreich: Klinische, mikrobiologische und epidemiologische Befunde. Tropcnmcd. Parasitol 2001; 23: 17-26. 14.Skarin A, Flor?n I, Kiss K et al. Acanthamoeba keratitis in the south of Sweden. ActaOphthalmologicaScandinavica 1996; 74(6), 593–597. 15.Radford CF, Minassian DC, Dart JKG. Acanthamoeba keratitis in England and Wales: incidence, outcome, and risk factors. Br J Ophthalmol. 2002; 86(5): 536–542. 16.Souza Alvarenga L, de Freitas D, Hofling-Lima AL. Ceratite por Acanthamoeba. ArqBrasOftal 2000; 63(2): 155-159. 17.Moore MB, McCulley JP, Kaufman HE et al. Radial keratoneuritis as a presenting sign in Acanthamoeba Keratitis. Ophthalmology 1986; 104: 1310-1315. 18.Seal DV. Acanthamoeba keratitis: update-incidence, molecular epidemiology and new drugs for treatment. Eye 2003; 17: 893-905. 19.Sharma S, Garg P, Rao GN. Patient characteristics, diagnosis, and treatment of non-contact lens related Acanthamoeba keratitis. Br J Ophthalmol 2000; 84: 1103-1108. 20.Sharma S, Srinivasan M, George C. Acanthamoeba keratitis in non-contact lens wearers. Arch Ophthalmol 1990; 108: 676-678. 21.Szentm?ry N, Goebels S, Matoula P et al. Die Akantham?benkeratitis - ein seltenes und oft sp?t diagnostiziertes Cham?leon. Klin Monatsbl Augenheilkd 2012; 229(5): 521-528. 22.Wright P, Warhust D, Jones BR. Acanthamoeba Keratitis successfully treated medically. Br J Ophthalmol 1985; 69: 778-782. 23.Bacon AS, Frazer DG, Dart JKG et al. A review of 72 consecutive cases of Acanthamoeba keratitis 1984-1992. Eye 1993; 7: 719-725. 24.Larkin DFP, Kilvington S, Dart JK. Treatment of Acanthamoeba keratitis with polyhexamethylenebiguanide. Ophthalmology 1992; 99: 185-191. 25.Bernauer W, Duguid GI, Dart JK. Zur klinischen Fr?hdiagnose der Acanthamoeben-Keratitis: Eine Studie ?ber 70 Augen. KlinMonatsblAugenheilkd 1996: 208(5); 282-284. 26.Dart JKG, Saw VPJ, Kilvington S. Acanthamoeba Keratitis: Diagnosis and Treatment Update 2009. Am J Ophthalmol 2009; 148: 487-499. 27.Del Buey MA, Crist?bal JA, Casas P et al. Evaluation of in vitro efficacy of combined riboflavin and ultraviolet A for Acanthamoeba isolates. Am J Ophthalmol 2012; 153(3): 399-404. 28.Khan YA, Kashiwabuchi RT, Martins SA et al. Riboflavin and ultraviolet light, a therapy as an adjuvant treatment for medically refractive Acanthamoeba keratitis: report of 3 cases. Ophthalmology 2011; 118: 324-331. 29.Mor?n H, Malmsj? M, Mortensen J, Ohrstr?m A: Riboflavin and ultraviolet A collagen crosslinking of the cornea for the treatment of keratitis. Cornea 2010; 29: 102-104. 30.Makdoumi K, Mortensen J, Crafoord S. Infectious keratitis treated with corneal crosslinking. Cornea 2010; 29(12): 1353-1358. 31.M?ller L, Thiel MA, Kipfer-Kauer AI and Kaufmann C. Corneal cross-linking as supplementary treatment option in melting keratitis: a case series. KlinischeMonatsblatterf?rAugenheilkunde 2012; 229(4): 411–415. |