J.ophthalmol.(Ukraine).2015;5:68-71.
|
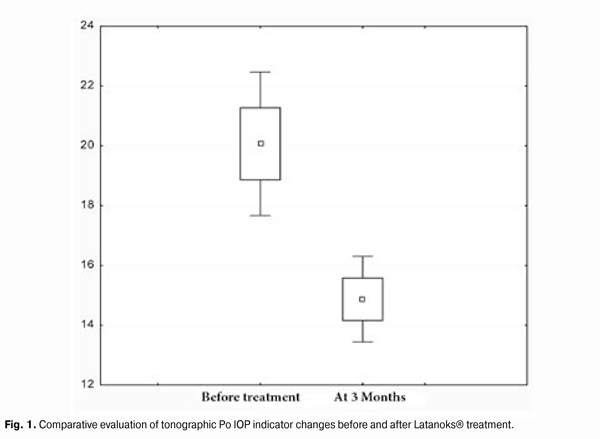
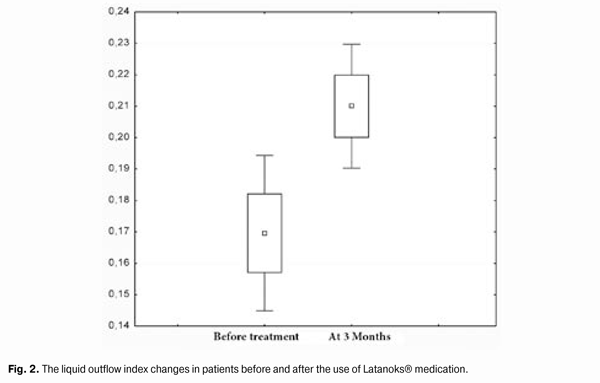
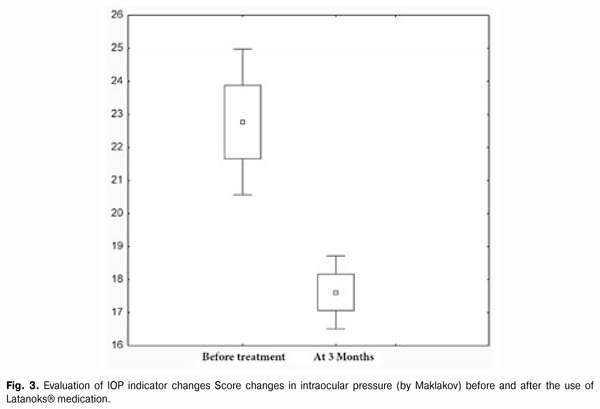
https://doi.org/10.31288/oftalmolzh201556871 Efficacy of Latanoks (latanaprost 0.005%) in patients with open-angle glaucoma Shambra SV, Doctor Korol AR, Doctor of Medical Sciences Filatov Eye Disease and Tissue Therapy Institute, the NAMS of Ukraine Odessa (Ukraine) E-mail: laserfilatova@gmail.com Introduction: Glaucoma refers to chronic diseases that require constant treatment. Conservative treatment of glaucoma involves the continuous assigning of local hypotensive medications for a long time to maintain normal range of intraocular pressure (IOP). Objective: To examine the efficacy of Latanoks medication in clinical practice with patients having primary open-angle glaucoma. Material and methods: 20 patients (30 eyes) have been under the observation with primary open-angle glaucoma stage I-III. All the patients were transferred to Latanoks monotherapy due to the lack of the intraocular pressure compensation. Tonometry, tonography, electronic static perimetry were performed at the beginning and the end of the observation period. Results: The use of Latanoks medication for the treatment of patients with an open-angle glaucoma within three months has led to a decrease of tonography Po index from an average (20.0 ± 6.0) mm Hg to (14.9 ± 3.0) mm Hg (p < 0.0003). The use of Latanoks for the treatment of patients with open-angle glaucoma within three months has led to a decrease of IOP (by Maklakov) from an average (22.8 ± 5.0) mm Hg. to (17.6 ± 2.0) mm Hg (p < 0.0002) and also has allowed to keep the visual field indicators stable for three months. Conclusion: Latanoks is an effective hypotensive medication that does authentically lower IOP and helps to save the visual field. Key words: glaucoma, tonometry, visual field, intraocular pressure
Introduction Primary glaucoma is a chronic progressive optic neuropathy. It is characterized by morphological changes in the optic nerve head and in the nerve fibers layer, progressive loss of ganglion cells of the retina and the emergence of specific defects in the vision field associated with these phenomena [1, 3]. Increased intraocular pressure (IOP) or its unstable values are the most important risk factors for the development and progression of glaucoma, because it contributes to the ganglion cells of the retina death and the axons of the optic nerve damage, leading to an irreversible blindness. Therefore, to preserve visual functions the primary objective is to reduce and stabilize an ophthalmotonous pressure. IOP decrease by 1 mm Hg reduces the risk of glaucoma process progression by 10%. And its decrease by 30% slows down significantly the speed of vision fields tunneling progression in patients with pseudonormal pressure glaucoma. There are a lot of potentialities to choose the medication for POAG therapy. Antiglaucoma medicines differ by their effect on hydrodynamics and hemodynamics. Today, these medications are divided into the first-line medications (prostaglandins analogues, ?-adrenergic antagonists) and the second-line medications (adrenergic agonists, carbonic anhydrase inhibitors) [2, 4]. According to the recommendations of the European Glaucoma Society, currently, in patients with open-angle glaucoma, the preferred use of prostaglandin medications for hypotensive monotherapy is considered to be reasonable. In the global ophthalmic practice, the pharmacological agents on the basis of latanoprost as an actual substance (F2a prostaglandines analogue [5]), have proven their efficacy. According to the data of clinical studies (Comparison of the Effects of Latanoprost and Timolol on Aqueous Humor Dynamics in Ocular Hypertensive Patients, University of Nebraska, USA; A 4-week, dose-ranging study comparing the efficacy, safety and tolerability of latanoprost 75, 100 and 125 ?g/mL to latanoprost 50 ?g/mL in the treatment of primary open-angle glaucoma and ocular hypertension), latanoprost does not significantly effect a cardiovascular and respiratory systems, being applied in therapeutic doses. Objective: To examine the efficacy of Latanoks medication in clinical practice with patients having primary open-angle glaucoma. Material and methods 20 patients (30 eyes) with primary open-angle glaucoma (POAG) stage I-III have been examined. They have been receiving the antiglaucoma treatment in the Department of Laser Microsurgery of the Eye of State Institution "The Filatov Institute of Eye Diseases and Tissue Therapy of the National Academy of Medical Sciences of Ukraine''. Prior their inclusion into the study, the patients has been receiving the hypotensive therapy with the use of ?-adrenergic blockers and cholinomimetics. At their inclusion to the study, the patients were transferred to Latanoks monotherapy (1 capsule per day for a period of 3 months without washout period. The main indication for Latanoks use was the lack of IOP compensation and/or visual field deterioration. All patients have underwent IOP measuring, electronic tonography, static perimetry 3 months before and after the treatment, as well as a control IOP measurement 2 weeks after Latanoks prescription. The tolerance of the medication was assessed by the patient's words and the conjunctival hyperemia was inspected under the slit lamp. During the evaluation of electronic tonography results we were interested the most in the values of so-called true intraocular pressure (Po) and outflow easiness index (C). Statistical processing of the results obtained was performed with the use of ''Statistica'' software. Standard deviation (?), differences certainty index (p) and Student criterion were assessed. Results and discussion 2 weeks after the beginning of Latanoks therapy, a decrease of IOP was observed as compared to its original level in all patients. Average Po index at the beginning of the study was (20.0 ± 6.0) mm Hg, 3 months after the use of Latanoks - (14.9 ± 3.0) mm Hg (p < 0.0003), (Fig. 1). Liquid outflow index indicator before the study was 0.17 ± 0.06. 3 months after the use of Latanoks it was 0.21 ± 0.04 (p < 0.0003), (Fig. 2). Average IOP level before the study was (22.8 ± 5.0) mm Hg. 3 month after the use of Latanoks it was (17.6 ± 2.0) mm Hg (p < 0.0002), (Fig. 3). Significant changes in indicators of visual fields have not been revealed: at the beginning of the study - (417.2 ± 79)°, and after the use of Latanoks ? (427.6 ± 81.7)°, (p < 0.03).
A good tolerance of Latanoks medication should be noted. It causes no discomfort during the treatment. Latanoks was characterized with mild conjunctival hyperemia at the beginning of treatment, which was subjectively revealed by the patients and by us during examination under the slit lamp. Our research data correspond to the literature data on more expressed hypotensive effect of latanoprost in the form of therapy when transferring the patients from ?-blockers or ?-blockers combination and cholinomimetics. Interaction of prostaglandins and cholinomimetics analogues in relation to the decrease of IOP remained controversial until recently. According to Bill A, Phillips CL. (1971), pilocarpine reduces an uveoscleral outflow. That was also noted in the study on the eyes with reduced trabecular outflow. Pilocarpine assignment was followed by paradoxical IOP increase (Bleiman BS, Schwartz AL, 1979). Comparative study of timolol maleate 0.5% solution efficacy, that was used 2 times a day, and latanoprost 0.005% solution used once daily, showed that the use of latanoprost leads to the decrease of ophthalmotonus to a greater extent in a larger number of patients. According to Alm A. et al. (1998), 56% of patients receiving latanoprost had lower IOP or it was equal to 17 mm Hg, and 27% of patients had it below 15 mm Hg. In the group of timolol maleate only 38% of patients had ophthalmotonus below 17 mm Hg and 14% had it lower than 15 mm Hg. Latanoprost has an effective impact on IOP level in patients with both POAG and closed-angle glaucoma (Chew PT. et al., 2002). According to Egorov EA, et al., and Diestelhorst M. et al., latanoprost monotherapy is more effective in relation to IOP reduction, than a combination of two medications: timolol and pilocarpine. Therefore, pending the combined therapy assignment it is recommended to assign the latanoprost monotherapy. According to O'Donoghue E, Sanchez JG, latanoprost monotherapy is more efficient, than the combined use of timolol and dorsolamide. IOP reduction in latanoprost group was 23% against the original level, whereas in timolol and dorsolamid group it was only 17% against the original level. When comparing the hypotensive effect of latanoprost and dorsolamide hydrochloride 2% solution three times a day, the decrease of ophthalmotonus to less than 18 mm Hg was achieved in 46% of patients receiving latanoprost and 9% of patients receiving dorsolamide (Hartleben C, 1999). Conclusions 1)The examined Latanoks medication (the eye drop of 2.5 ml per dropper-vial produced by «Jadran» (Croatia) is effective for decreasing IOP and satisfactorily tolerable among patients with open-angle glaucoma diagnosis. 2)The use of Latanoks® medication for the treatment of patients with open-angle glaucoma during 3 months has allowed keeping the indicators of vision fields stable. 3)Latanoks may be recommended to be used in patients with open-angle glaucoma as monotherapy in the absence of IOP compensation by ?-blockers or combination of ?-blockers and cholinomimetics. References 1.Egorov EA. [Hypotensive treatment of glaucoma]. Klinicheskaya oftalmologiya. 2000; 1: 6-10. In Russian. 2.Egorov EA, Romanova OV. [Perspectives of latanoprost prostaglandin F2 Alpha analogue in hypertensive glaucoma therapy]. Vest oftalmol. 1998; 114 (4): 19-20. In Russian. 3.Erichev VP. [Main directions of hypotensive treatment of patients with primary glaucoma]. Rus. oftalm. zhur. 2000; 1(1): 18-21. In Russian. 4.Nesterov AP. [General glaucoma treatment methods evaluation and selection]. Fiziologia i patologiya vnutriglaznogo davleniya. 1987; 60-68. In Russian. 5.European Glaucoma Society Terminology & Guidelines for Glaucoma (European Directive). Glaucoma Society. 2nd ed. Savona Italy: DOGMA Editrice. 2003; 3 (R): 3-26. 6.Aoyama Y. Effect of UF-021 (Rescula (r)), a prostaglandin-related compound on intraocular pressure and anterior chamber flare counts (in Japanese). Ueno s. Folia. Ophthalmol. J. 1996; 47: 914-919. 7.Aquino MV, Lat-Luna M. The effect of latanoprost vs timolol on intraocular pressure in patients with glaucoma and ocular hypertension. Asian j. Ophthalmol. 1999; 1(R): 3-7. 8.Azuma I, Masuda K, Kitazawa Y. et al. Double-masked comparative study of UF-021 and timolol ophthalmic solutions in patients with primary open-angle glaucoma or ocular hypertension. Jpn. J. Ophthalmol. 1993; 37(4): 514-525. 9.Cardascia N, Vetrugno M, Trabucco T, Cantatore F, Sborgia C. Effects of travoprost eye drops on intraocular pressure and pulsatile ocular blood flow: a 180-day, randomized, double-masked comparison with latanoprost eye drops in patients with open-angle glaucoma. Current Therapeutic Research: Clinical and Experimental. 2003; 63: 389-400. 10.Chew PT, Hung PT, Aung T. Efficacy of latanoprost in reducing intraocular pressure in patients with primary angle-closure glaucoma. Surv. Ophthalmol. 2002; 47(1): 125-128. 11.David R. Changing Therapeutic paradigms in glaucoma management. Expert. Opin. Investing drugs.1998; 7:1063-1086. 12.Diestelhorst M, Nordmann JP, Toris CB. Combined therapy of pilocarpine or latanoprost with timolol versus latanoprost monotherapy. Surv. Ophthalmol. 2002; 47(1): 155-161. 13.Dubiner HB, Sircy MD, Landry T, Bergamini MV. W, Silver LH, Turner FD, Robertson S, Andrew RM, Weiner A, Przydryga J. Comparison of the diurnal ocular hypotensive efficacy of travoprost and latanoprost over a 44-hour period in patients with elevated intraocular pressure. Clinical Therapeutics. 2004.; 26: 84-91. 14.Easthope SE. Topical bimatoprost: a review of its use in open-angle glaucoma and ocular Hypertension. Perry CM. Drugs Aging. 2002; 19: 231-248. 15.Fellman RL, Sullivan EK, Ratliff M. et al. Comparison of travoprost 0.0015% and 0.004% with timolol 0.5% in patients with elevated IOP: a six- month, masked, multicenter trial. Ophthalmology. 2002; 109: 998-1008.
|