J.ophthalmol.(Ukraine).2015;4:28-34.
|
https://doi.org/10.31288/oftalmolzh201542834 Amino acids in the vitreous and intravitreal fluid in rhegmatogenous retinal detachment patients with different proliferative vitreoretinopathy grades G.V. Levitskaya, Cand. Sc. (Med) Filatov Eye Disease and Tissue Therapy Institute, Odessa E-mail: g.levytskaya@mail.ru Background:Proliferative vitreoretinopathy (PVR) is known to worsen the treatment prognosis of rhegmatogenous retinal detachment (RRD) and to result in irreversible loss of vision due to apoptotic or necrotic process in retinal neurons. Some studies have tried to examine the role of amino acids (first of all, neurotransmitters) in the development of proliferative vitreoretinopathy (PVR). Glutamate is known to cause migration and proliferation of RPE and/or glial cells. Based on this fact, it has been hypothesized that antagonists of glutamate may prevent or reduce the intensity of these processes. Additionally, it has been proved that elevated vitreous glutamate levels in proliferative diabetic retinopathy are potentially toxic to retinal ganglion cells, and that elevated vitreous levels of gamma-aminobutyric acid and glutamate promote the development of vasoproliferative processes. In general, little is still known about the role of immune acids and that of the imbalance in their levels in the pathogenesis of retinal pathology, and the importance of the issue of PVR complications is of no doubt. Purpose: To investigate the features of the distribution of amino acids in the vitreous and intravitreal fluid in patients with RRD with different PVR grades. Materials and Methods: Eighty-eight patients (88 eyes) were ofthalmologically examined and underwent surgery for RRD. Gas-liquid chromatography was utilized to determine the levels of 15 amino acids in vitreous and intravitreal fluid samples obtained at the time of vitrectomy and fluid-gas exchange to provide tamponade, respectively. Data were analyzed taking into account the PVR grade (grades A, B and C in 11, 64 and 13 patients, respectively). Results: In RRD patients, the levels of amino acids under study in the vitreous and, especially, in intravitreal fluid increased significantly with the increase in PVR grade to grade B. If the PVR continued to progress to grade C, a tendency to decrease in the neurotransmitter levels was observed, but they remained statistically significantly higher than those in grade A. Significant correlations were revealed between PVR grade and amino acid levels in intravitreal fluid, and, in most cases, in the vitreous. Further progression of the proliferative process was accompanied by a higher increase in the levels of excitatory amino acids (aspartate and glutamic acid), than that of the inhibitory one (glycine). Conclusion: The data presented provide evidence for the role of elevated amino acid (first of all, neurotransmitter) levels in the development of proliferative processes in RRD. The imbalance found in the levels of excitatory and inhibitory neurotransmitters (aspartate and glutamic acid vs. glycine) results in the excitotoxic effect, and creates pathochemical preconditions for retinal degeneration. Keywords: rhegmatogenous retinal detachment, proliferative vitreoretinopathy, amino acids, neurotransmitters, intravitreal fluid, vitreous. Introduction Because proliferative vitreoretinopathy (PVR) is characterized by the formation of membranes on both surfaces of the detached retina and is a potentially dangerous pathological process leading to irreversible loss of vision, the importance of the issue of PVR complications in the course of different ocular diseases is of no doubt. Nevertheless, up to the present time, no comprehensive, unified theory has been build up to explain the causes of the emergence and progression, and the mechanism of the development of proliferative processes [1]. They can develop following different penetrating ocular injuries related to trauma or surgery (e.g. vitrectomy or other intraocular surgery). The main factor to initiate and trigger proliferative complications is the breakdown of the blood?ocular barrier with the following inflammatory response (migration and proliferation of pigment epithelial cells, fibroblasts, myofibroblasts, and endothelial cells that form the cell membranes). In proliferative complications, the final products are fibrovascular glial membranes distorting the retinal surface and caused by the proliferative and glial tissue growth along the posterior hyaloids surface and preretinal hemorrhages [2]. In general, any intraocular pathologic process (including that resulted from surgical trauma) that causes migration of the retinal pigment epithelium (RPE) can result in the development of PVR [3]. Some studies have tried to examine the role of amino acids (first of all, neurotransmitters) in the development of proliferative vitreoretinopathy (PVR). Thus, the levels of gamma-aminobutyric acid (GABA), glutamate, and vascular endothelial growth factor (VEGF) have been examined in the vitreous of patients with proliferative diabetic retinopathy [4]. The levels of GABA and glutamate in the vitreous of patients with PDR, 29.4 ± 7.8 mumol/L and 24,7±14,0 mumol/L, were 2.0 and 2.5 times, respectively, higher than those in non-diabetic controls (P = 0.004 and P < 0.001, respectively). Vitreous VEGF level was also significantly higher in patients with PDR (1759 ± 1721 pg/mL) compared with controls (27 ± 65 pg/mL) (P < 0.001). Moreover, moderately strong correlations were found between GABA and VEGF levels (r = 0.68) and glutamate and VEGF levels (r = 0.43). Elevated GABA, glutamate, and VEGF levels have been shown to be correlated strongly with the presence of PDR. Therefore, Ambati et al [5] have proved that elevated vitreous levels of glutamate in PDR are potentially toxic to retinal ganglion cells. Elevated vitreous GABA may reflect amacrine cell dysfunction and underlie electroretinographic oscillatory potential abnormalities seen in diabetic retinopathy. Additionally, in PDR, elevated vitreous GABA in the presence of elevated vitreous VEGF level promotes the development of neovascularization that is actually a response to tissue ischemia. Glutamate is known to cause migration and proliferation of RPE and/or glial cells. Based on this fact, it has been hypothesized that antagonists of glutamate may prevent or reduce the intensity of these processes. Therefore, avoidance or management of PVR can be achieved by administering to the patient a compound capable of reducing glutamate-induced retinal cell migration in a concentration effective to reduce such migration [3]. Because PVR plays a significant role in the development of complications of rhegmatogenous retinal detachment (RRD), and little is known on the presence and levels of amino acids in ocular media and fluids in different clinical manifestations of PVR, the purpose of the study was to investigate the features of the distribution of amino acids in the vitreous and intravitreal fluid in patients with rhegmatogenous retinal detachment with different proliferative vitreoretinopathy grades.
Materials and Methods A total of 88 RRD patients (88 eyes; age, 21 to 74 years) that had undergone vitrectomy with gas tamponade to achieve retinal reattachment were included into the study. All patients underwent assessment of the level of visual acuity, ocular tonometry, refractometry, biomicroscopy, indirect ophthalmoscopy, and optical coherent tomography (OCT). The main clinical characteristics of retinal detachment were extent (within 6 clock hours in 15 eyes and more than 6 hours in 73 eyes), height (moderately high and high retinal detachment in 34 eyes and 54 eyes, respectively) and duration (within 10 days in 34 eyes and more than 10 hours in 81 eyes). In 35 vitreous samples and 67 intravitreal fluid samples [6], gas-liquid partition chromatography was utilized to determine the levels of the following amino acids as was described previously [7]: alanine, arginine, aspartate, valine, histidine, glutamic acid, glycine, isoleucine, leucine, lysine, proline, serine, tyrosine, threonine, and phenylalanine. Vitreous samples were obtained at the time of vitrectomy, whereas intravitreal fluid samples were obtained 1 to 2 days following vitrectomy, at the time of repeat fluid-gas exchange to provide additional tamponade in order to increase the volume of vitreous cavity gas bubble for adequate prevention of inferior retinal breaks [7]. The intervention was performed using the standard approach, with the patient in a seated position: after the needle was inserted at 4 (3.5) mm from the limbus at the 6 o’clock position, 0.1 to 0.5 mL of intravitreal fluid was aspirated and simultaneous injection of the gas-and-air mixture into the ocular cavity was performed. Amino acid content in the vitreous and that in intravitreal fluid in RRD patients was analyzed taking into account the PVR grade (grade A in 11 patients, and grades B and C in 77 patients (64 and 13 patients, respectively)). Statistical analysis was performed using Statistica 6.0 software. If there was a normal distribution, a parametric t test was used for pairwise comparison of the sample means between two groups. The nonparametric Kruskal-Wallis test was utilized to compare differences between more than two groups. Spearman rank correlation was used to assess correlation between the parameters [8].
Results and Discussion The results of the analysis of amino acid levels in the vitreous and those in intravitreal fluid in RRD patients are presented in Table 1, with the PVR grades taken into account. In all patients with PVR grades B and C, increased amino acid levels were found in the media evaluated. Moreover, in the majority of these patients, these levels were statistically significantly higher than those in patients with PVR grade A. We have shown previously that the amino acid levels in intravitreal fluid are higher than those in the vitreous [7]. With the increase in the severity of PVR, the increase in the amino acid levels in intravitreal fluid was found to be more apparent than that in the vitreous. Table 1. Amino acid levels in RRD patients with different PVR grades (ng/mL)
Note. n, number of eyes; P, significance level. Spearman correlation analysis revealed (Table 2) significant correlations between PVR grade and amino acid levels in intravitreal fluid, and, in most cases, in the vitreous (excluding valine, isoleucine, and tyrosine, with rho = 0.274, rho = 0.278 and rho = 0.347, respectively, and P > 0.05). Table 2. Spearman rank correlation between amino acid levels in the vitreous and intravitreal fluid and PVR grade in rhegmatogenous retinal detachment
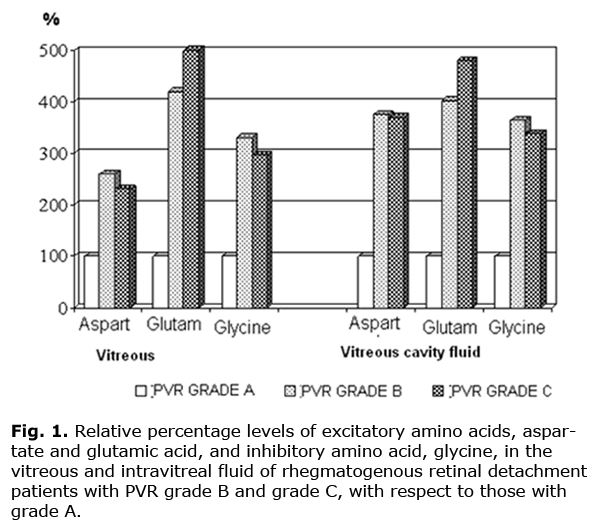
Note. n, number of eyes; P, significance level. Because PVR is one of the most important signs characterizing the clinical course of rhegmatogenous retinal detachments, we analyzed additionally the neurotransmitter levels in eyes with PVR grade A, grade B and grade C (11, 64 and 13 eyes, respectively). A Kruskal–Wallis test showed a significant effect of the PVR grade on the vitreous glutamic acid level (chi-square = 6.306, df = 2, P = 0.0427). Additionally, the test revealed an increasing trend in vitreous aspartate and glycine levels (chi-square = 2.307, df =2, P = 0.316, and chi-square =3.162, df =2, P = 0.206, respectively) across PVR grade A, grade B and grade C groups. Moreover, the test showed that the increase in the amino acid levels in intravitreal fluid (i.e., in the material obtained 1 to 2 days following retinal reattachment surgery) was statistically significant in all the cases (e.g., chi-square = 15.516, df = 2, P = 0.0004 for glutamic acid, chi-square = 11.738, df =2, P = 0.0028 for aspartate, and chi-square =11.738, df = 2, P = 0.0028 for glycine). The analysis of the neurotransmitter levels revealed statistically significant relationships between the PVR grade and aspartate, glutamic acid, and glycine levels in intravitreal fluid (with rho = 0.373, rho =0.585 and rho =0.397, and P = 0.002, P = 0.000 and P = 0.001, respectively, and n = 67), and between the PVR grade and glutamic acid levels in the vitreous (with rho = 0.520, P = 0.001, and n = 35). Fig.1 shows neurotransmitter levels in the vitreous and intravitreal fluid of RRD patients with PVR grades B and C with respect to those with PVR grade A. It is well seen that with the increase in the grade of PVR, the increase in the content of all these acids was observed. Thus, the vitreous levels of excitatory amino acids, aspartate and glutamic acid, were 2.64 and 4.22 times increased in the PVR grade B compared to PVR grade A, and 2.35 and 5.06 times increased in the PVR grade C compared to PVR grade A. The vitreous levels of the inhibitory amino acid, glycine, were 3.38 times and 3.02 times increased in the PVR grade Band PVR grade C, respectively, versus PVR grade A. It should be noted that, the differences in both inhibitory and excitatory amino acid levels in intravitreal fluid between the patients with different PVR grades were more marked. Thus, the levels of aspartate and glutamic acid were 3.74 and 4.81 times increased in the PVR grade B compared to PVR grade A, and 3.69 and 4.04 times increased in the PVR grade C compared to PVR grade A. The levels of glycine were 3.64 times and 3.40 times increased in the PVR grade Band PVR grade C, respectively, versus PVR grade A. Therefore, analysis of amino acid levels taking into account the PVR grade showed that these levels change significantly with the increase from grade A to grade B. If the PVR continued to progress, with the development of fixed retinal folds (grade C), and if the detachment duration was significant, a tendency to decrease in the neurotransmitter levels was observed. However, the levels remained statistically significantly higher than those in the lowest PVR grade (grade A) (Fig. 1).
Additionally, the data obtained provide evidence that further progression of the proliferative process is accompanied by the increase in the levels of both excitatory and inhibitory amino acids, with the changes of the latter being less apparent. This was confirmed by the corresponding values of the coefficient estimated as the ratio between the sum of the excitatory amino acid indices and that of the inhibitory acid indices: these values for the vitreous samples in PVR grade A, grade B and grade C were 5.67, 6.03 and 7.51, respectively, versus 3.99, 4.70 and 4.55 for the samples of intravitreal fluid. Conclusion The development of proliferative vitreoretinopathy in RRD patients is accompanied by an increase in the levels of neurotransmitters, aspartate, glutamic acid, and glycine, both in the vitreous and intravitreal fluid. If the PVR continues to progress, with the development of fixed retinal folds, and if the detachment duration is significant, a tendency to decrease in the neurotransmitter levels is observed. In RRD patients with progressive PVR, an imbalance between the levels of excitatory and inhibitory neurotransmitters (aspartate and glutamic acid vs. glycine) is observed, resulting in the excitotoxic effect, and creating pathochemical preconditions for retinal degeneration.
References 1. Medzhidova SR. [Posttraumatic proliferative vitreoretinopathy: Literature Review].Oftalmologiya. 2012;10(3):113-20. Russian. 2. Zapuskalov IV, Krivosheina OI. [Current concept of the pathogenesis of proliferative vitreoretinopathy]. In: [Proceeding of the Conference on New Technologies for the Treatment of Vitreoretinal Pathology]; 2006 Mar 23-24; Moscow (Russia): Eye Microsurgery Federal State Institution; 2006. p.72-76. Russian. 3. Dreyer EB, inventor; Allergan Sales, Inc., assignee. Calcium blockers to treat proliferative vitreoretinopathy. United States patent US 20050192322 A1. 2005 Sept 1. 4. Ambati J, Chalam KV, Chawla DK et al. Elevated gamma-aminobutyric acid, glutamate, and vascular endothelial growth factor levels in the vitreous of patients with proliferative diabetic retinopathy. Arch. Ophthalmol. 1997; 115 (9):1161–6. 5. Abdullaieva EA, Saidova LKh, Zeinalova IF. [Clinical significance of antiangiogenic therapy of a subretinal neovascular membrane in complicated myopia]. Oftalmologiya. 2013;11(1):57-63. Russian. 6. Levitskaya GV. [Potential to minimize surgery for rhegmatogenous retinal detachment]. In: [Proceeding of the 9th Conference on New Technologies for the Treatment of Vitreoretinal Pathology]; 2011 Mar 10-11; Moscow (Russia): Eye Microsurgery Federal State Institution; 2011. p.123-125. Russian. 7. Levitskaya GV. [Amino acid spectrum in vitreous body and vitreous content in patients with retinal detachment]. Rus Det Oft. 2014;1:16-20. Russian. 8. Glantz SA. Primer of Biostatistics. 4th ed. [Transl. from English]. Moscow: Praktika;1998. 459 p. Russian. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||