J.ophthalmol.(Ukraine).2015;4:11-16.
|
https://doi.org/10.31288/oftalmolzh201541116 Role of the complex enzyme immunoassay testing in diagnosing uveitis in the presence of herpetic infection S.R. Medjidova, Candidate of Medical Science, Acad. Z. Alieva National Center of Ophthalmology, Baku, Azerbaijan E-mail: sabmed@rambler.ru; sabinamedjidova@gmail.com Background: In recurrent uveitis, establishing whether any herpetic infection has played an etiological or triggering role in the disease is important for selecting the appropriate treatment. Purpose: To assess the results of complex enzyme immunoassay testing (CEIAT) performed for patients with both uveitis and herpes simplex virus (HSV) infection in the process of treatment. Materials and Methods: In 73 uveitis patients, the CEIAT was used to determine serum levels of anti?HSV-IgМ and anti?HSV-IgG antibodies (Ab) to herpes simplex virus type 1 and type 2 (HSV1&2), IgG class Ab to nonstructural early viral antigens of HSV1&2, and the avidity of specific IgG Ab to HSV1&2. Results: The most common etiology of uveitis was herpetic infection (25.7%). In 74.3% of cases, HSV played a triggering role. The following three types of specific immunity were registered: (1) significantly (р<0.01) increased levels of anti-HSV IgM and anti?HSV-IgG Ab and IgG class Ab to nonstructural early viral antigens, with the avidity index (AI) values exceeding 50% (27 patients), (2) the most increased (р<0.001) levels of anti?HSV-IgG Ab and IgG class Ab to nonstructural early viral antigens, with the AI values exceeding 50% (35 patients), and (3) only the level of IgG class Ab to nonstructural early viral antigens is slightly increased (р<0.05), with high AI values (12 patients). The third type of immune response was as a manifestation of the immune tolerance of the human body, with the most clinically severe course of the inflammatory process. Conclusion: In uveitis in the presence of HSV infection, the results of the CEIAT provide the practitioner an opportunity to investigate the activity of inflammatory process and define a pathogenetically sound scheme to correct the alterations revealed. Keywords: uveitides, herpes simplex virus, complex enzyme immunoassay testing.
Background The worldwide incidence and prevalence of herpes simplex virus (HSV) infection is increasing year by year. Nearly 80-90% of the global population are HSV-infected. HSV has a variety of clinical presentations and paths of infection, and is capable of initiating a chronic pathological process with regular exacerbations [1]. Given the social character of the problem of a global infection with HSV, the WHO Regional Office for Europe has grouped the disease with the most threatening infectious pathologies in this century. Primary HSV infection generally occurs in early childhood and may remain for life clinically unapparent. Reactivation of latent infection can be influenced by various exogenous and endogenous factors leading to the failure of the immune system to protect the body against infection [2]. In an ocular inflammatory pathology, herpes viruses can assume the role of etiologic factor (ophthalmic herpes) or that of a trigger for the recurrence of ocular inflammatory process (herpes-associated ophthalmic pathology). Durable replicative activity of herpes viruses in the tissues of the damaged organs is a risk factor for the recurrence of herpetic disease [3]. Establishing the etiological diagnosis of the anterior ocular inflammatory disease caused by HSV infection alone generally is not a particular problem for the ophthalmic practitioner [4]. However, in recurrent central and posterior uveitis, establishing whether any herpetic infection has played an etiological or triggering role in the disease is important for selecting the appropriate treatment. This also results from numerous reports on the pronounced herpes-specific humoral immunity in uveitis in the presence of systemic and syndromic diseases [5,6]. The management of such cases requires a meticulous approach allowing for the optimal selection of medications in need [7,8]. The purpose of the study was to assess the results of complex enzyme immunoassay testing (CEIAT) performed for patients with both uveitis and herpes simplex virus (HSV) infection in the process of treatment. Material and Methods The study involved 74 patients (age range, 21-57 years; 41 males and 33 females) with uveitis in the presence of herpetic infection. The share of patients aged 24 to 49 years was 78.4% (58 patients). Clinical and laboratory examination methods were used. The clinical examination involved history taking, visual acuity measurement, refractometry, tonometry, biomicroscopy, fundus ophthalmoscopy, A-scan and B-scan ocular ultrasonography, and optical coherence tomography (OCT) and fluorescein fundus angiography (FFA) (provided the refractive media were transparent). In this paper, we refer to the data from Standardization of Uveitis Nomenclature (SUN) Project developed by the three international organizations (American Uveitis Society, International Uveitis Study Group (IUSG), and International Ocular Inflammation Society) that study the pathogenesis of, and produce novel diagnostic and treatment modalities for uveitis, and implement the blindness-prevention measures. At baseline (time 0, before initiating the treatment) and one month after clinical recovery, all the patients were subjected to complex laboratory examination for the following purposes: to determine the etiology of the disease, investigate whether allergic sensibilization and associated viral infection were present, assess the immune status during the treatment process, and schedule the date for the next examination. With this in mind, clinical blood and urine chemistry tests were performed, and basic coagulograms were taken. Additionally, blood samples were assayed for the levels of glucose, C-reactive protein, rheumatoid factor, antistreptolysin, fibrinogen, serological reactions to different infections, immune status, allergic hypersensitization indices (total IgE), presence of antinuclear antibodies (ANA), and myeloperoxidase antibodies and proteinase 3 antibodies (MPO-ANCA, PR3-ANCA). All assay kits were used as per the manufacturers' instructions. Given the potential for a trigger role of herpetic infection, and in addition to the above-mentioned tests, all patients were subjected to the complex enzyme immunoassay testing for this infection. Modern enzyme-linked immunosorbent assays (ELISA) were used to determine serum levels of specific antibodies against HSV1&2 (anti?HSV-IgМ, anti?HSV-IgG, Euroimmun, Germany), IgG class antibodies to nonstructural early viral antigens, and the avidity of the specific IgG antibodies to HSV1&2. The serum level of IgG antibodies to nonstructural early viral antigens was utilized as a reactivation marker. Since this approach detects serum IgG antibodies to nonstructural early antigens of the viruses, it is particularly valuable for the assessment of reactivated chronic infection at the onset of clinical illness [9]. Serum samples were examined with a solid-phase ELISA assay (Bioservice Biotechnology Company Limited, Russia) as per the manufacturer's instructions. Purified recombinant polypeptides, the analogs of the following non-structural viral proteins were absorbed to the bottom of the wells of 96-well plates: a basic early HSV-1 and HSV-2 DNA-binding protein, and a reference antigen – the polypeptide synthesized in E.coli cells and containing no antigenic determinants of herpes viruses. Pre-diluted (1:10) serum samples were introduced into the plate wells and the plates were incubated at 37?C for 60 minutes. Serum HSV-specific antibodies form immune complexes with the antigens absorbed to the bottom of the wells. After the fluid was aspirated, the wells were washed three times to remove unbound antibodies, and HSV-specific horseradish peroxidase (HRP)-conjugated antibodies were introduced into the wells to detect the formed specific solid-phase immune complexes. The plates were incubated at 37?C for 60 minutes and washed to remove unbound antibodies. Chromogen solution was added to the wells to stain the formed complexes, the plates were kept for 15 minutes at room temperature in a dark chamber, and the stop reagent was added to the wells. The color intensity (i.e., optical absorbance (OA)) was measured spectrophotometrically at 492 nm. The OA for each well was proportional to the level of antibodies to nonstructural viral antigens in the sample. The score of each serum sample (?OA) was calculated as the difference between the OA value of the examined serum sample with HSV antigens and the OA value of the examined serum sample with the reference antigen. If the ?OA value was higher than 0.350, the serum sample was considered HSV-positive (i.e. the person had serological markers of HSV activity). The avidity of the specific IgG antibodies to HSV1&2 was assessed with “IFA-antiHSV-1+2-IgG-avidity” assay kit (ECOlab, Russia). Avidity refers to the binding strength between the antibody and its specific antigen, and depends on the number of binding sites and the binding force.The immune response to an invading agent consists of production of specific IgM antibodies and specific IgG antibodies by the stimulated lymphocyte clone.IgG antibodies produced early in infection have low avidity.High-avidity antibodies develop several weeks and even months later, have stronger binds with antigens and can eliminate these antigens more reliably.Since HSV-specific antibodies have high avidity, a recent primary infection can be ruled out.The avidity index (AI) for HSV1&2-specific IgG can be used to determine the age of infection more accurately and discriminate between primary infection and acute exacerbation of chronic infection or latent infection. The principle of the kit is based on the fact that species-specific IgG binds to the antigen absorbed to the wells of the polystyrene plate.The complexes formed from the pathogen antigen and low-avidity specific IgG dissociate when acted upon by dissociation solution, with the degree of dissociation depending on the IgG avidity for the antigen.Undissociated complexes bind with HRP-conjugated antibodies against human IgG.After adding the indicator solution, the color of the plate well reaction mixture changes proportional to the level of species-specific HRP-conjugated antibodies.The color development is stopped with the addition of stop solution.The color intensity (i.e., optical absorbance) of the sample is measured spectrophotometrically.The AI (in percent) is calculated by using the following formula: AI = (ODsample+DS / ODsample+PBS-T) x 100, where ODsample+DS is the optical density for the well with dissociation solution, ODsample+PBS-T is the optical density for the well with phosphate buffered saline (25X) with Tween. We considered AI values of <45% low avidity and AI values of >50% high avidity; AI values between 45% and 50% were considered intermediate avidity. Excel-2007 software was used for statistical analyses. The method of variation statistics was used in processing the data obtained, and arithmetic means and extreme values of a sample were computed. Student's t-test was used to assess the significance of the data obtained. Results In the patients examined, the following four types of uveitis were registered with regard to the damage location (as per international classification made by SUN Working Group): anterior uveitis (39 patients; 52.7%), intermediate uveitis (6 patients; 8.1%), posterior uveitis (20 patients; 27%) and pan-uveitis (9 patients;12.2%) (Fig. 1).
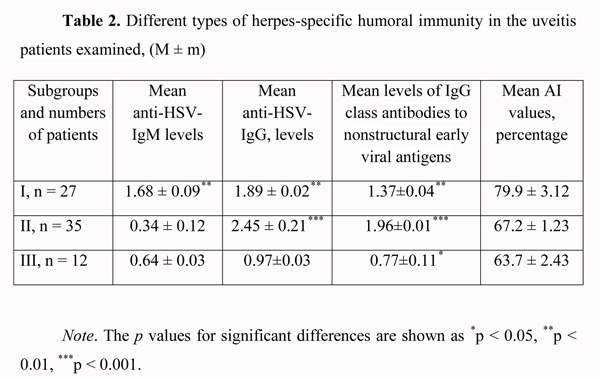
As one can see, the largest share was that of anterior uveitis patients. Bilateral and unilateral uveitis were found in 21 patients (28.4%) and 53 patients (71.6%), respectively. The course of disease was favorable in 28 patients (37.8%), and chronic recurrent in 46 patients (62.2%). Each of the latter patients had 2-3 recurrent episodes, 6 months to 18 months apart. Acute and subacute choroidal inflammation processes were found in 34 patients (45.9%) and 40 patients (54.1%), respectively. A comparative causative analysis of choroidal damage in the patients of the study was used to found the etiologic role of herpes virus infection in 19 cases (25.7%) out of 74 patients. In Table 1, we present the etiology of choroidal inflammation in other 55 cases (74.3%); in these cases, a high titer of antiviral antibodies was found along with the basic disease. Additionally, in these cases, a viral infection presented an unfavorable background for the management of basic disease and could have a triggering role in the recurrence of ocular inflammatory process. One can see from the summary of the etiological analysis that most commonly (25.7%), the major cause of uveitis was herpetic infection. However, a trigger role of herpes virus infection creates some problems in the management of patients with this infection along with any autoimmune or systemic disease (rheumatoid arthritis, ankylosing spondylitis, Beh?et's disease, and Vogt-Koyanagi-Harada disease) requiring immune suppressive therapy (corticosteroids, cytostatic agents). Therefore, we performed the above-mentioned enzyme immunoassay testing to analyze the serological changes in antiherpetic immunity in these patients. In the patients examined, we registered the following three types of herpes-specific humoral immunity (Table 2). (1)
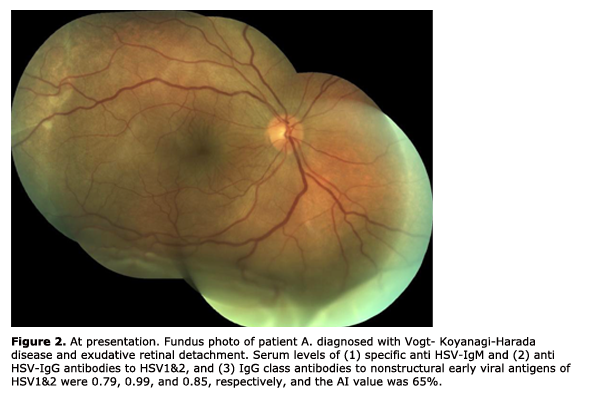
In 27 patients (36.5%), there was a significant (р < 0.01) increase in the levels of anti-HSV IgM (1.68 ± 0.09) and anti?HSV-IgG (1.89 ± 0.02) antibodies and IgG class antibodies to nonstructural early viral antigens (1.37 ± 0.04), and the AI values exceeded 50% (79.9 ± 3.12%). (2) In 35 patients (47.3%), the level of anti?HSV-IgМ antibodies was insignificantly higher (р > 0.05) than normal values (0.34 ± 0.12), whereas the levels of anti?HSV-IgG (2.45 ± 0.21) and IgG class antibodies to nonstructural early viral antigens were significantly higher (р < 0.001) than critical values (1.96 ± 0.01), and the AI values exceeded 50% (67.2 ± 1.23%). (3) In 12 patients (16.2%), the level of anti?HSV-IgМ antibodies was not significantly higher (р > 0.05) than normal values (0.64 ± 0.03), the level of anti?HSV-IgG was registered within the grey zone (0.97 ± 0.03), whereas the level of IgG class antibodies to nonstructural early viral antigens was slightly increased (0.77 ± 0.11, р < 0.05), thus evidencing reactivation of herpetic infection in the presence of increased AI values (63.7±2.43%). The first two types of immune response are the evidence of exacerbation of chronic herpetic infection. We found the most favorable type of the clinical course of the inflammatory process (mean duration, 10-14 days) in the first subgroup, and a more prolonged one (mean duration, 13-18 days) in the second subgroup. The third type of immune response may be interpreted as an unfavorable manifestation of some kind of immune tolerance of the human body, with the most clinically severe course of the inflammatory process (mean duration, 20-27 days). This type of immune response was found in 2, 3, 2, 3, 1 and 1 patients with herpetic uveitis, rheumatoid arthritis, ankylosing spondylitis, Beh?et's disease, diabetes mellitus, and Vogt-Koyanagi-Harada disease (Fig. 2, 3), respectively. When the disease in these patients was in a quiescent state, we adjusted their initial therapy by co-administration of immunomodulators (Imunofan, 1.0 ml administered as intramuscular injections every other day, 10 injections for a course). In all three subgroups, a month after treatment, the AI values (73.4 ± 3.51%, p < 0.01) and the level of IgG class antibodies to nonstructural early viral antigens (1.79 ± 0.41, p < 0.01) exceeded critical values.
Conclusions In uveitis, herpetic infection can play not only an etiological role; in some cases it can play a triggering role, thus aggravating the course of the basic disease. In patients with uveitis in the presence of herpetic infection, the complex enzyme immunoassay testing (i.e., determining the serum levels of specific anti?HSV-IgМ and anti?HSV-IgG antibodies to HSV1&2, IgG class antibodies to nonstructural early viral antigens, and the avidity of the specific IgG antibodies to HSV1&2) allows to investigate the activity of the inflammatory process, and perform the comparative analysis of clinical and immunological indices of this activity and immune response in the quiescent and acute phases of the disease.In uveitis, the results of the complex immunoenzymatic testing for herpetic infection will provide the practitioner with an opportunity to define a pathogenetically sound scheme to correct the alterations revealed, thus improving the selection of appropriate medications in therapeutically difficult cases of systemic and autoimmune diseases. References 1. Kuskova TK, Belova EG. [Current opinion on the herpes virus family]. Lech Vrach. 2004;(5):6-11. Russian. 2. Obukhova OO. [Pathogenetic features of inflammation and methods to correct inflammation in the quiescent and acute phases of chronic herpetic infection]. Abstract of a Thesis for the Degree of Doctor of Medical Science. Novosibirsk, 2009. 32 p. Russian. 3. Dolgikh TI. [Potential of modern laboratory diagnostics for infectious diseases: methods, algorithms, and interpretation of the results]. Tutorial for physicians. Omsk: Omsk State Medical Academy; 2005. 40 p. Russian. 4. Rustamova NM. [Differential diagnosis of uveitis of various etiologies]. Abstract of a Thesis for the Degree of Candidate of Medical Science. Baku, 2000. 20 p. 5. Mal’chikov IA. [Importance of viral infections in a pathology related to altered protection against infection and methods of their detection]. Abstract of a Thesis for the Degree of Doctor of Medical Science. Ekaterinburg, 2007. 47 p. Russian. 6. Safina AZ. [Role of herpes viruses, mycoplasmas and chlamydia in rheumatoid arthritis and treatment with cyclopheron]. Thesis for the Degree of Candidate of Medical Science. Ufa, 2004. 101 p. Russian. 7. Drozdova EA, Tarasova LN. [Diagnostics and management of uveitis in rheumatic diseases]. Tutorial. Moscow; 2012. 95 p. Russian. 8. Rougier MB, Isber R, Colin J et al. The treatment of refractory uveitis with human intravenous immunoglobulin. Ophthalmic Res. 2001;33(5):73. 9. Krichevskaia GI, Maichuk IuF, Vakhova ES. [Method of prognosing the risk of recurrent ocular herpes-associated diseases]. Patent dated 02.11.2007. Russian. |