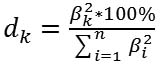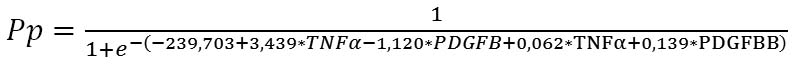J.ophthalmol.(Ukraine).2020;1:3-9.
|
http://doi.org/10.31288/oftalmolzh2020139 Received: 24 December 2019; Published on-line: 21 February 2020 Predicting post-surgical recurrent diabetic maculopathy in patients with type 2 diabetes mellitus Iu.O. Panchenko1, Cand Sc (Med); S.Yu. Mogilevskyy2, Dr Sc (Med), Prof.; S.V. Ziablitsev3, Dr Sc (Med), Prof 1 Kyiv Municipal Clinical Hospital “Eye Microsurgery Center”; Kyiv (Ukraine) 2 Shupik National Medical Academy of Postgraduate Education; Kyiv (Ukraine) 3 Bohomolets National Medical University;Kyiv (Ukraine) E-mail: sergey.mogilevskyy@gmail.com TO CITE THIS ARTICLE: Panchenko IuO, Mogilevskyy SYu, Ziablitsev SV. Predicting post-surgical recurrent diabetic maculopathy in patients with type 2 diabetes mellitus. J.ophthalmol.(Ukraine).2020;1:3-9.http://doi.org/10.31288/oftalmolzh2020139
Background: A closed subtotal vitrectomy (CSV), either alone or in combinations with internal limiting membrane (ILM) peeling, panretinal laser coagulation (PRLC); and cataract phacoemulsification (phaco) is a common treatment for diabetic maculopathy (DMP). Predicting post-surgical recurrent DMP in patients with type 2 diabetes mellitus (T2DM) based on identified pathogenetic factors for T2DM, and genotyping, medical history and clinical findings is a challenge. Purpose: To develop a model for predicting post-surgical recurrent DMP in patients with T2DM. Material and Methods: The study included 313 patients with T2DM (313 eyes) and DMP. Patients received one of the four types of surgical treatment: only a 25+ three-port CSTV (n=78); CSTV combined ILM peeling in a 2.5 mm- to 3.5-mm diameter macular area (n=85); CSTV combined with ILM peeling and panretinal laser coagulation (PRLC) (n=81); or CSTV combined with ILM peeling, PRLC and phaco (n=69). Preoperatively, ELISA was used to determine blood endothelin-1 (ET1) and tumor necrosis factor alpha (TNF?), and platelet-derived growth factor (PDGF-BB) levels, and polymorphisms of PDGFB (rs1800818) and TNF? (rs1800629) were investigated by real-time polymerase chain reaction. Follow-up visits were at months 1, 3, 6 and 12 after surgery. Multivariate logistic regression analysis was used to develop a model in Statistica 10 (GLZ; StatSoft, Tulsa, OK, USA). Results: The regression analysis showed no association of age, gender, severity and duration of T2DM, glucose and glycated hemoglobin (НbA1c) levels, diabetic retinopathy (DR) stage and severity on the ETDRS scale, central retinal thickness (CRT0), or blood ЕТ1 level with post-surgical recurrent DMP within 12 months after surgery. Genotypes of TNF-? (rs1800629) and PDGFB (rs1800629) polymorphisms, and blood PDGF-BB and TNF? levels were found to be significant predictors of the presence or absence of recurrent DMP. The probability of developing recurrent DMP was directly related to the TNF? rs1800629 polymorphism and blood PDGF-BB and TNF? levels, and inversely related to the PDGFB rs1800818 polymorphism. The percentage contributions of selected independent variables in predicting the dependent variable were found to be as follows: TNF? rs1800629, 90.25%; PDGFB rs1800818, 9.57%; PDGF-BB, 0.15%; and TNF?, 0.03%. Performance measures of the model were satisfactory, with Area under Curve (AUC) = 0.993; -2*log (Likelihood) = 197.80; ?2 = 110.15 (p < 0.001). Conclusion: TNF-? (rs1800629) and PDGFB (rs1800629) polymorphisms, and blood PDGF-BB and TNF? levels were predicting factors for recurrent DMP within 12 months after surgery. Keywords: diabetic maculopathy, type 2 diabetes mellitus, surgical treatment, recurrence, rs1800629, rs1800818, PDGF-BB, TNF?
Introduction In patients with type 2 diabetes mellitus (T2DM), diabetic maculopathy (DMP) refractory to conservative, laser and/or anti-VEGF therapy is treated with a version of subtotal closed vitrectomy (STCV), either alone, or, if required, combined with panretinal laser coagulation (PRLC) or, if indicated, with cataract phacoemulsification (CPE) [1, 2]. Vitrectomy is effective for DMP, and the effect can be increased by additional peeling off the internal limiting membrane (ILM) without an increase in intra- or postoperative complications [3]. No significant difference in postoperative visual acuity was found between patients with non-tractional macular edema who underwent vitrectomy with and without ILM peeling [4, 5]. Studies vary in the reported incidence, timing and patterns of recurrence after various combinations of surgical procedures for DMP [1, 3, 5-7]. Previously, we have reported that the annual incidence of recurrence after surgery for DMP was 29.7%. In addition, at 1, 3, 6 and 12 months after surgery, 23.0%, 18.2%, 10.2% and 24.9%, respectively, of patients had recurrent DMP. Moreover, there was no statistically significant difference in the recurrence rate between various surgical techniques. However, the efficacy of surgical treatment for DMP decreased with increase in severity of diabetic retinopathy (DR), and annual incidence of recurrence after surgery in mild non-proliferative diabetic retinopathy (NPDR), moderate or severe NPDR, and proliferative diabetic retinopathy (PDR) was 27.5%, 22.6%, and 33.7%, respectively [7, 8]. Therefore, developing systems for predicting DMP recurrence after surgery in patients with T2DM is important. Some pathophysiological biomarkers of DR are prognostic factors of value for this purpose [9, 10]. Previously, we have found that endothelial dysfunction markers, endothelin-1 (ET1) and tumor necrosis factor alpha (TNF?), as well as platelet-derived growth factor (PDGF-BB) are risk factors for recurrent DMP [11]. It is noteworthy that some clinical signs, e.g., disease duration ? 6 years, moderate-to-severe course with switching over to insulin therapy, obesity, and arterial hypertension have been identified by others as risk factors for DMP after surgery [12, 13]. We believe it is reasonable to consider clinical factors (such as gender, age, duration and severity of T2DM and DR), and the polymorphisms related to the development of visual disease in DM2 [13] as potential factors for predicting recurrent DMP. The purpose of the study was to develop a model for predicting post-surgical recurrent DMP in patients with T2DM. Material and Methods The study included 313 patients with T2DM (313 eyes) and DMP. These included patients with mild nonproliferative diabetic retinopathy (NPDR; Group 1; n=40), moderate or severe NPDR (Group 2; n=92), and proliferative diabetic retinopathy (PDR; Group 3; n=181). Each patient underwent an eye examination which included visual acuity assessment, static Humphrey perimetry, refractometry, tonometry, slit lamp biomicroscopy, gonioscopy, ophthalmoscopy with Volk Super Field lens and Goldmann three-mirror lens (Volk Optical, Mentor, OH). In addition, spectral domain optical coherence tomography (SD-OCT; Copernicus REVO, Optopol Technology Sp, zo.o, Zawiercie, Poland; scan programs, Retina 3D and Retina Raster) and OCT (Retina Angio mode) were performed. We identified the presence of the true decorrelation signal from blood flow within the preretinal vitreous layer in OCTA images for identification of initial vitreoretinal neovascularization and areas of capillary occlusion (ischemia) in the superficial and deep retinal vascular plexuses. Fundus photography (the ETDRS seven standard fields) was performed with the fundus camera TRC-NW7SF (Topcon, Tokyo, Japan). Fluoresecein angiography (FA) was performed with the fundus camera if (a) mild vitreoretinal neovascularization or proliferation was suspected but not identified with ophthalmoscopy or fundus photography or (b) the visual function did not correspond either to ophthalmoscopic changes in the macula or OCT findings. Severity of DR and DMP was graded as per the 2002 guidelines of the American Academy of Ophthalmology [1]. Each patient underwent SCV for DMP refractory to conservative, laser and/or anti-VEGF therapy. Preoperatively, ELISA was used to determine blood PDGF-BB, TNF? and ЕТ1 levels with Human PDGF-BB Quantikine ELISA Kit (R&D Systems; USA), and kits from Bender Medsystems, and Biomedica Immunoassays (Austria), respectively. Polymorphisms of PDGFB (rs1800818; locus, 22q13.1; location, intron of 5?-UTR Variant) and TNF? (rs1800629; -308G/A; location, 6p21.33) were investigated by real-time polymerase chain reaction (PCR). At the first phase of the study, DNA extraction from venous blood was done using the Invitrogen™ PureLink Genomic DNA kit for purification of genomic DNA (Invitrogen Inc.) following the manufacturer’s instructions. At the second phase of the study, real-time PCR was conducted using using TaqMan Mutation Detection Assays (Life Technologies, Carlsbad, CA;) and Gene Amp® 7500 PCR System (Applied Biosystems, Foster City, CA). Ninety-five age- and sex-matched individuals without ocular pathology or T2DM were used as controls. The study design and protocol were approved by the ethics committee of Shupik National Medical Academy of Postgraduate Education and adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from all patients. Patients received one of the four types of surgical treatment: only 25+ three-port closed subtotal vitrectomy (CSTV; n=78); CSTV combined with internal limiting membrane (ILM) peeling in a 2.5 mm- to 3.5-mm diameter macular area (n=85); CSTV combined with ILM peeling and panretinal laser coagulation (PRLC) (n=81); and CSTV combined with ILM peeling, PRLC and cataract phacoemulsification (phaco) (n=69). Follow-up visits were at months 1, 3, 6 and 12 after surgery. The rate of recurrent DMP after surgery was determined. Multivariate logistic regression analysis was used to develop a model in Statistica 10 (GLZ; StatSoft, Tulsa, OK, USA). A set of independent variables for the development of regression equations was formed based on the principle of availability and adequacy of the data that can be obtained in the initial period of patient observation at enrollment in the study. These variables were as follows: age, gender, severity and duration of T2DM, degree of hyperglycemia compensation as assessed by glucose and glycated hemoglobin (НbA1c) levels, DR stage and severity on the ETDRS scale, central retinal thickness (CRT0), blood levels of ЕТ1, TNF?, and PDGF-BB, and genotypes of PDGFB rs1800818 (“101”, “102”, and “103” were used as dichotomous values for wild homozygote, heterozygote, and minor homozygote, respectively). In the first phase of the development of multivariate regression models, significant predictors were selected from the set of independent variables based on the estimation of beta coefficients regarding the significance of their difference from the null hypothesis (p < 0.05). The following formula was used to calculate the percentage effect of the selected regression characteristics on the dependent variable:
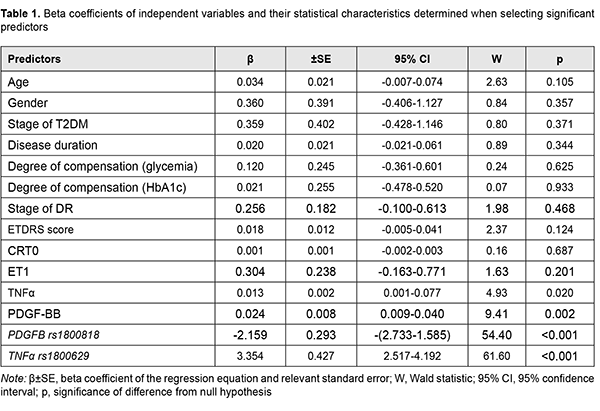
where dk is the percentage effect of the characteristic; ?k is beta coefficient of the independent variable of regression equation; and the term of the fraction represents the sum of squares of beta coefficients for all independent variables Results and Discussion The nominal variable determining the presence or absence of recurrent DMP within 12 months after surgery was used as a dependent variable in model development. Dichotomous values (“1” for the presence, and “0” for the absence of recurrent DMP) were used for this variable in the regression equation. The four factors effecting recurrent DMP (blood TNF?, blood PDGF-BB, PDGFB rs1800818 and TNF? rs1800629 genotypes) were selected as significant predictors for the regression equation for predicting recurrent DMP (Table 1).
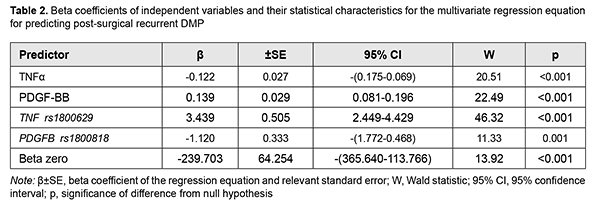
A number of studies have been conducted on specific values of these factors (TNF?, PDGF-BB, and polymorphisms of their genes) for T2DM and its complications [14-29]. The role of TNF? in the development of insulin resistance due to diminishing tyrosine phosphorylation of insulin receptor substrate (IRS)-1 and thus suppressing further activation of PI3K/Akt and ERK/MAP kinase pathways and uptake of glucose has been demonstrated [14, 15, 16]. In addition, through activation of intracellular mediating proteins (RIPK1, BIRC2 and BIRC3), TNF? activates TNF-receptor, NFkB, Toll-like receptor and apoptosis signaling pathways, which also has a role in the development of insulin resistance [17]. Meta-analyses found that TNF-? 308G>A polymorphism was strongly associated with T2DM risk in Han Chinese population [18], and TNF-? -308 "A" allele conferred significant susceptibility towards both T2DM and diabetic complications, and was shown to influence the serum cytokine levels (cytokine storm) under diabetic conditions in Indian populations [19, 20]. A Brazilian study [21] demonstrated that the A allele of the –308G>A polymorphism was more frequent in subjects with PDR than in those with no DR, and independently associated with an increased risk of PDR. The correlation between the TNF-? promoter genotypes and the risk of developing T2DM remains controversial due to numerous discrepancies between the different studies available. Since ethnic differences may play a role in these conflicting results [22], separate studies should be performed for different ethnic populations. Previously, we have demonstrated the pathogenic value of PDGF in DMP [23]. PDGF-ВВ is produced by activated macrophages and neoangiogenic endothelium in hypoxia and ischemia, stimulates endothelial cell proliferation and neoangionenesis, and increases capillary permeability [24]. Hyperglycemia causes post-receptor resistance to PDGF through activation of protein kinase C (PKC)-delta, MAP kinase and protein tyrosine phosphatase-1 (SHP-1), leading to PDGF receptor-? (PDGFR?) dephosphorylation, which also increases apoptosis of vascular pericytes [25]. It has been shown that PDGF-B/ PDGFR? signalling is critical in formation and maturation of blood-retinal barrier (BRB) through active recruitment of pericytes onto growing retinal vessels. In addition, impaired pericyte recruitment to the vessels showed multiple vascular hallmarks of diabetic retinopathy (DR) due to BRB disruption [26]. In a study by Praidou and colleagues [27], the levels of all PDGF isoforms in the vitreous were significantly increased in the PDR group, as compared to controls, but no such differences were evidenced in serum. In addition, a PDGFR-? knockout mice study demonstrated that PDGF-BB-PDGFR? and PDGFR? axes are potential therapeutic targets for the prevention of tractional retinal detachment [28]. Therefore, certain data in the literature support the results of the regression analysis. It is the gene factors and, to a lesser extent, the regulator factors depending on these gene factors, that caused post-surgical recurrent DMP. Based on the obtained results, we developed a four-factor regression model for predicting post-surgical recurrent DMP. Table 2 presents beta coefficients for independent variables, beta zero and relevant statistic characteristics for the multivariate regression equation for predicting post-surgical recurrent DMP.
Given the absolute values of beta coefficients, the TNF? rs1800629 (the predictor with the beta coefficient (|3.439|) with largest absolute value) was the greatest contributor to the probability of developing recurrent DMP, followed by the PDGFB rs1800818 (|-1.120|), PDGF-BB (|0.139|) and TNF? (|0.062|). The probability of developing recurrent DMP was directly related to the TNF? rs1800629 polymorphism and blood PDGF-BB and TNF? levels, and inversely related to the PDGFB rs1800818 polymorphism. Because these relationships reflected the patterns that we have reported previously [11, 23] (elevated blood PDGF-BB and TNF? levels were associated with the recurrent DMP), the probability of the recurrence of DMP is directly related to the values of these levels. The minor allele of rs1800629 and the ancestor allele of rs1800818 were associated with the recurrence. Therefore, the former polymorphism increases, whereas the latter decreases the probability of recurrent DMP, which is shown by the signs of beta coefficients. The percentage contributions of selected independent variables in predicting the dependent variable (formula 1) were found to be as follows: TNF? rs1800629, 90.25%; PDGFB rs1800818, 9.57%; PDGF-BB, 0.15%; and TNF?, 0.03%. The regression equation for predicting the probability of postsurgical DMP recurrence in patients with T2DM is as follows:
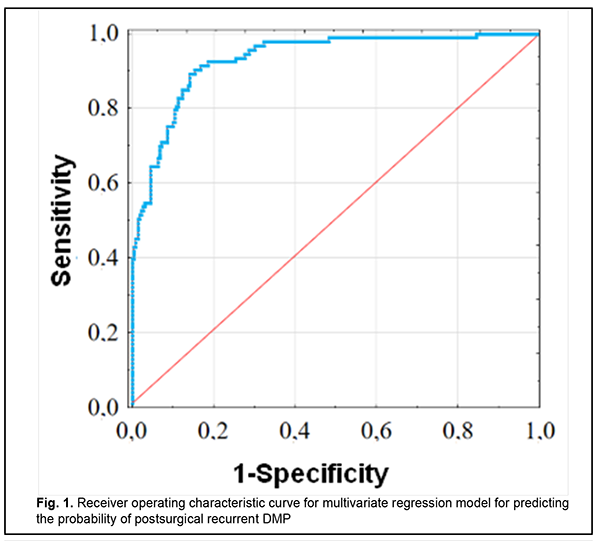
where Rp is the probability of postsurgical DMP recurrence; TNF? is the dichotomous value for the rs1800629 genotype; PDGFB is the dichotomous value for the rs1800818 genotype; TNF? is the blood TNF? level expressed in pg/mL; and PDGFBB is the blood PDGF-BB level expressed in ng/mL ROC analysis was used to calculate performance measures of the model (Fig. 1). Performance measures of the model were satisfactory, with Area under Curve (AUC) = 0.993; -2*log (Likelihood) = 197.80; ?2 = 110.15 (p < 0.001).
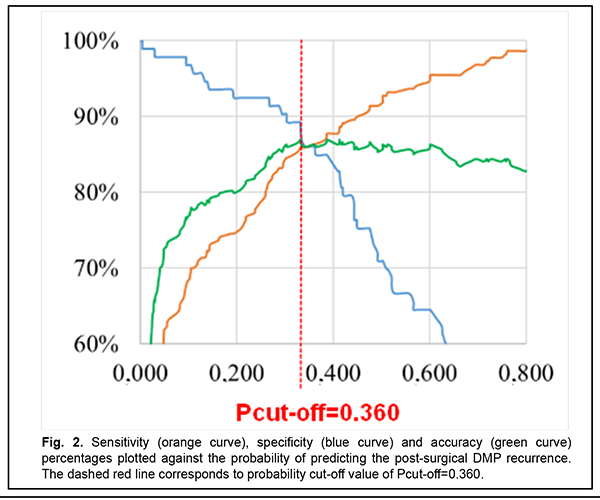
The diagram (Fig. 2) (1) shows relationships of the probability of developing recurrent DMP and sensitivity (orange curve), specificity (blue curve), and accuracy (green curve) of the developed regression model, and (2) was used to determine the optimum probability for discriminating between negative and positive predictions. Based on the accuracy characteristic analysis, the probability cut-off was set to Pcut-off=0.360.
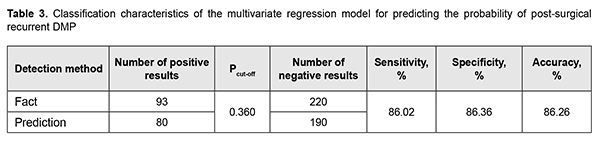
Table 3 presents classification characteristics of the regression model for the selected Pcut-off value.
Predictions of recurrent DMP using formula 2 have an accuracy of 86.3%. Conclusion First, genotypes of TNF-? (rs1800629) and PDGFB (rs1800629) polymorphisms, and blood PDGF-BB and TNF? levels were found to be significant predictors of the presence or absence of recurrent DMP within 12 months after surgery. Second, the percentage contributions of selected independent variables in predicting the dependent variable were found to be as follows: TNF? rs1800629, 90.25%; PDGFB rs1800818, 9.57%; PDGF-BB, 0.15%; and TNF?, 0.03%. Finally, a regression model for predicting the probability of postsurgical recurrent DMP in patients with T2DM was developed. Performance measures of the model were satisfactory, with AUC = 0.993; -2*log (Likelihood) = 197.80; and ?2 = 110.15 (p < 0.001).
References 1.Balashevich LI, Izmailov AS. [Diabetic ophthalmopathy]. St. Petersburg: Chelovek; 2012. Russian. 2.Xiao K, Dong YC, Xiao XG, Liang SZ, Wang J, Qian C, Wan GM. Effect of pars plana vitrectomy with or without cataract surgery in patients with diabetes: a systematic review and meta-analysis. Diabetes Ther. 2019 Oct;10(5):1859-1868. doi: 10.1007/s13300-019-0672-9. 3.Hu XY, Liu H, Wang LN, Ding YZ, Luan J. Efficacy and safety of vitrectomy with internal limiting membrane peeling for diabetic macular edema: a Meta-analysis. Int J Ophthalmol. 2018 Nov 18;11(11):1848-55. doi: 10.18240/ijo.2018.11.18. 4.Jackson TL, Nicod E, Angelis A, Grimaccia F, Pringle E, Kanavos P. PARS PLANA VITRECTOMY FOR DIABETIC MACULAR EDEMA: A Systematic Review, Meta-Analysis, and Synthesis of Safety Literature. Retina. 2017 May;37(5):886-95. doi: 10.1097/IAE.0000000000001280. 5.Rinaldi M, dell'Omo R, Morescalchi F, Semeraro F, Gambicorti E, Cacciatore F, Chiosi F, Costagliola C. ILM peeling in nontractional diabetic macular edema: review and metanalysis. Int Ophthalmol. 2018 Dec;38(6):2709-14. doi: 10.1007/s10792-017-0761-6. 6.Norihito Doi, Taiji Sakamoto. Comparative study of vitrectomy versus intravitreous triamcinolone for diabetic macular edema on randomized paired-eyes. Graefes Arch Clin Exp Ophthalmol. 2012;250:71-8. 7.Panchenko Yu. Effectiveness off different methods of surgical treatment of diabetic maculopathy in patients with type 2 diabetes. American Science Journal. 2019;28(2):27-34. 8.Panchenko Yu. Possibilities and effectiveness of cataract phacoemulsification, closed subtotal vitrectomy and panretinal laser coagulation in diabetic maculopathy treatment in patients with type 2 diabetes. East European Science Journal. 2019;7(47 part 2):50-6. 9.Eshaq RS, Aldalati AMZ, Alexander JS, Harris NR. Diabetic retinopathy: Breaking the barrier. Pathophysiology. 2017;24(4):229-41. 10.Jenkins AJ, Joglekar MV, Hardikar AA, Keech AC, O'Neal DN, Januszewski AS. Biomarkers in Diabetic Retinopathy. Rev Diabet Stud. 2015 Spring-Summer;12(1-2):159-95. 11.Mogilevskyy S.Iu., Panchenko Iu.O., Ziablytsev S.V. New risk factors for post-surgical recurrent diabetic maculopathy in type 2 diabetus mellitus. Journal of Ophthalmology (Ukraine). 2019;5(490):9-17. 12.Kamoi K, Takeda K, Hashimoto K, Tanaka R, Okuyama S. Identifying risk factors for clinically significant diabetic macula edema in patients with type 2 diabetes mellitus. Curr Diabetes Rev. 2013 May;9(3):209-17. 13.Diep TM, Tsui I. Risk factors associated with diabetic macular edema. Diabetes Res Clin Practice. 2013 Jun;100(3):298-305. 14.Liu C, Feng X, Li Q, Wang Y, Li Q, Hua M. Adiponectin, TNF-? and inflammatory cytokines and risk of type 2 diabetes: A systematic review and meta-analysis. Cytokine. 2016 Oct;86:100-109. doi: 10.1016/j.cyto.2016.06.028. 15.IRS1 – Insulin receptor substrate 1 – Homo sapiens (Human) – IRS1 gene & protein". www.uniprot.org. Retrieved 2016-04-21. 16.Takaguri A. [Elucidation of a new mechanism of onset of insulin resistance: effects of statins and tumor necrosis factor-? on insulin signal transduction]. Yakugaku Zasshi. 2018;138(11):1329-1334. doi: 10.1248/yakushi.18-00116. Japanese. 17.Jamil K, Jayaraman A, Ahmad J, Joshi S, Yerra SK. TNF-alpha -308G/A and -238G/A polymorphisms and its protein network associated with type 2 diabetes mellitus. Saudi J Biol Sci. 2017 Sep;24(6):1195-1203. doi: 10.1016/j.sjbs.2016.05.012 18.Liu ZH, Ding YL, Xiu LC, Pan HY, Liang Y, Zhong SQ, Liu WW, Rao SQ, Kong DL. A meta-analysis of the association between TNF-? -308G>A polymorphism and type 2 diabetes mellitus in Han Chinese population. PLoS One. 2013;8(3):e59421. doi: 10.1371/journal.pone.0059421. 19.Dhamodharan U, Viswanathan V, Krishnamoorthy E, Rajaram R, Aravindhan V. Genetic association of IL-6, TNF-? and SDF-1 polymorphisms with serum cytokine levels in diabetic foot ulcer. Gene. 2015 Jul 1;565(1):62-7. doi: 10.1016/j.gene.2015.03.063. 20.Doody NE, Dowejko MM, Akam EC, Cox NJ, Bhatti JS, Singh P, Mastana SS. The role of TLR4, TNF-? and IL-1? in type 2 diabetes mellitus development within a North Indian population. Ann Hum Genet. 2017 Jul;81(4):141-146. doi: 10.1111/ahg.12197. 21.Sesti LF, Crispim D, Canani LH, Polina ER, Rheinheimer J, Carvalho PS, Gross JL, Santos KG. The -308G>A polymorphism of the TNF gene is associated with proliferative diabetic retinopathy in Caucasian Brazilians with type 2 diabetes. Invest Ophthalmol Vis Sci. 2015 Jan 29;56(2):1184-90. doi: 10.1167/iovs.14-15758. 22.Luna GI, da Silva IC, Sanchez MN. Association between -308G/A TNFA Polymorphism and Susceptibility to Type 2 Diabetes Mellitus: A Systematic Review. J Diabetes Res. 2016;2016:6309484. 23.Mogilevskyy SIu, Panchenko IuO, Ziablytsev SV. Predicting the risk of diabetic retinopathy-assosiated macular edema in patients with type 2 diabetes mellitus. Journal of Ophthalmology (Ukraine). 2019;3(488):3-8. 24.Mori K, Gehlbach P, Ando A, Dyer G, Lipinsky E, Chaudhry AG, Hackett SF, Campochiaro PA. Retina-specific expression of PDGF-B versus PDGF-A: vascular versus nonvascular proliferative retinopathy. Invest Ophthalmol Vis Sci. 2002;43:2001-6. 25.Geraldes P, Hiraoka-Yamamoto J, Matsumoto M, Clermont A, Leitges M, Marette A, Aiello LP, Kern TS, King GL. Activation of PKC-delta and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nature Med. 2009;15:1298-306. 26.Park DY, Lee J, Kim J, Kim K, Hong S, Han S, Kubota Y, Augustin HG, Ding L, Kim JW, Kim H, He Y, Adams RH, and Koha GY. Plastic roles of pericytes in the blood-retinal barrier. Nat Commun. 2017 May 17;8:15296. 27.Praidou A, Klangas I, Papakonstantinou E, Androudi S, Georgiadis N, Karakiulakis G, Dimitrakos S. Vitreous and serum levels of platelet-derived growth factor and their correlation in patients with proliferative diabetic retinopathy. Curr Eye Res. 2009 Feb;34(2):152-61. 28.Kitahara H, Kajikawa S, Ishii Y, Yamamoto S, Hamashima T, Azuma E, Sato H, Matsushima T, Shibuya M, Shimada Y, Sasahara M. The Novel Pathogenesis of Retinopathy Mediated by Multiple RTK Signals is Uncovered in Newly Developed Mouse Model. EBioMedicine. 2018 May;31:190-201.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|