J.ophthalmol.(Ukraine).2019;6:44-48.
|
http://doi.org/10.31288/oftalmolzh201964448 Received: 20 September 2019; Published on-line: 06 January 2020 Stabilization of glaucoma after non-perforating deep sclerectomy with laser trabeculoplasty ab externo L. Rudavska, MD; I. Novytskyy, Dr Sc (Med), Prof. Danylo Halytsky Lviv National Medical University; Lviv (Ukraine) E-mail: lidiarud91@gmail.com TO CITE THIS ARTICLE: Rudavska L, Novytskyy I. Stabilization of glaucoma after non-perforating deep sclerectomy with laser trabeculoplasty ab externo. J.ophthalmol.(Ukraine).2019;6:44-48. http://doi.org/10.31288/oftalmolzh201964448
Background: Non-perforating deep sclerectomy (NPDS) is an efficacious and safe procedure for the treatment of glaucoma. Purpose: To examine the effect of NPDS alone vs NPDS with a simultaneous laser trabeculoplasty (LT) ab externo in patients with primary open-angle glaucoma (POAG). Material and Methods: Patients of group 1 (94 patients; 94 eyes) underwent a combined procedure of NPDS and LT ab externo, whereas those of group 2 (control group; 80 patients; 80 eyes) underwent NPDS alone for POAG. Results: In group 1, mean intraocular pressure (IOP) before surgery and at month 12 and month 24 was 27.1 ± 2.2 mm Hg, 19.1 ± 1.2 mm Hg and 19.8 ± 2.6 mm Hg, respectively, vs 26.9 ± 2.0 mm Hg, 20.6 ± 1.5 mm Hg, and 21.5 ± 1.2 mm Hg, respectively, in group 2. In addition, in the combination procedure group, mean Mean Deviation (MD) before surgery and at month 12 and month 24 was -8.1 ± 6.9 dB, -8.5 ± 6.9 dB, and -8.9 ± 7.0 dB, respectively, vs -8.8 ±7.6 dB, -9.2 ± 7.6 dB, and -9.7 ± 7.4 dB, respectively, in the control group. Moreover, in group 1, mean retinal nerve fiber layer (RNFL) thickness before surgery and at month 12 and month 24 was 65.3 ± 16.5 nm, 64.2 ± 16.3 nm and 63.6 ± 15.9 nm, respectively, in the combination procedure group, vs 67.1 ± 14.9 nm, 65.8 ± 15.2 nm and 62.3 ± 15.4 nm, respectively, in group 2. Conclusion: NPDS with a simultaneous LT ab externo slows the progression of glaucomatous optic neuropathy. Keywords: primary open-angle glaucoma, intraocular pressure, optic disc, non-perforating deep sclerectomy, laser trabeculoplasty, static automated perimetry, optical coherence tomography
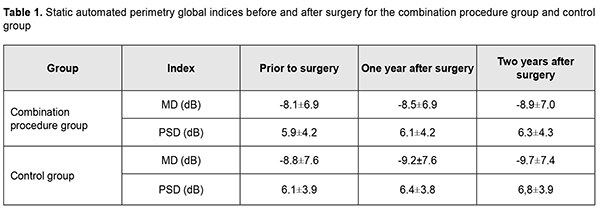
Introduction Non-perforating deep sclerectomy (NPDS) is the safest filtration surgery for primary open-angle glaucoma (POAG). However, the efficacy of the technique may be compromised due to decreased filtration through the juxtacanalicular trabecular meshwork that remained intact. Identifying the approaches that will improve the efficacy of NPDS is important for ophthalmologists. The purpose of the study was to examine the effect of NPDS alone versus NPDS with a simultaneous laser trabeculoplasty ab externo in patients with POAG. Material and Methods This study involved 174 patients (174 eyes; 86 men and 88 women; mean age, 64.5 ± 7.3 years) with POAG. They underwent an eye examination including visual acuity assessment, tonometry, tonography, static perimetry, and optical coherence tomography (OCT) of the optic disc and peripapillary retinal nerve fiber layer (RNFL). The intraocular pressure (IOP) was measured with the 10-gram Maklakov tonometer preoperatively, at week 1, month 1, and every 3 months thereafter under conditions of a compensated IOP. A simplified version of Nesterov technique was used to perform tonography. The course of glaucomatous optic neuropathy was assessed based on changes in global retinal sensitivity, variability of visual field defects, and results of OCT studies of optic disc morphometric parameters and RNFL thickness. Patients underwent static perimetry using Oculus Twinfield perimeter (Oculus Inc, Wetzlar, Germany) with a 24–2 SITA standard program. The results of the visual field test in this study were reliable if fixation loss was less than 20% and false-positive and false-negative rates were less than 25%. A decrease in global retinal sensitivity (mean deviation, MD) correlates with a decrease in optic media transparency. MD and pattern standard deviation (PSD) were used to assess the functional progression of POAG. Static perimetry was performed every four months with a mean follow-up of 26.1 ± 2.3 months. Morphometric optic disc analysis as well as RNFL thickness assessment was performed with the SD-OCT system (3D-OCT 1000 Mark II; Topcon, Tokyo, Japan). The anatomic criteria for glaucoma progression were RNFL thickness and morphometric optic disc parameters, vertical cup-to-disc ratio and neuroretinal rim area. RNFL thickness was determined at 256 points at a 3.4-mm diameter around the center of the optic disc during a single scan, and these values were averaged for each eye. OCT was performed twice a year. Hoddap-Parrish-Anderson criteria [1] for glaucomatous visual field changes were used to diagnose glaucoma. Early, moderate and severe visual field defects were found in 74 eyes (42.5%), 67 eyes (38.5%), and 33 eyes (18.9%), respectively. Patients were divided into two groups. Patients of group 1 (94 patients; 94 eyes) underwent a combined procedure of NPDS and micropulse diode laser trabeculoplasty ab externo [2], whereas patients of group 2 (controls; 80 patients; 80 eyes) underwent NPDS alone. The indication for surgery was intolerance to anti-glaucoma medications or failure to achieve the target IOP despite the maximum use of tolerated anti-glaucoma medication. Mean preoperative IOP was 27.1 ± 2.2 mm Hg for group 1 and 26.9 ± 2.0 mm Hg for group 2. All patients received the same type of hypotensive therapy before surgery. Combinations of prostaglandins and beta-blockers only were administered in 156 eyes (89.6%), and combinations of prostaglandins and beta-blockers with carbonic anhydrase inhibitors or alpha-2 adrenergic agonists, in 15 eyes (8.6%). Three patients (1.7%) received monotherapy (prostaglandins). Mean number of hypotensive medications being administered preoperatively was 2.3 ± 0.2 for group 1 and 2.0 ± 0.2 for group 2. Complete success of surgery was defined as a Maklakov IOP of ?22 mm Hg without the use of anti-glaucoma medications. Partial success of surgery was defined as a Maklakov IOP of ?22 mm Hg with the use of anti-glaucoma medications. Failure of surgery was defined as a Maklakov IOP of >22 mm Hg with the use of anti-glaucoma medications. Results In patients of group 1, mean IOP decreased by 10.0±0.7 mm Hg (36.9%) to 17.1±0.8 mm Hg (р <0.001) at day 7, and was 17.9±1.1 mm Hg without the use of medications at month 1. At month 12, the number of patients in the combination procedure group decreased to 79 (79 eyes) due to drop out, and mean IOP was 19.1±1.2 (р <0.05). In addition, of these 79 (79 eyes), 57 (72.1%) had a Maklakov IOP of <22 mm Hg without the use of anti-glaucoma medications, 13 (16.5%) used topical anti-glaucoma medications, and 9 (11.4%) had a Maklakov IOP of >22 mm Hg with the use of anti-glaucoma medications. Mean number of hypotensive medications for achieving the target IOP was 0.6±0.1 for group 1. At month 24, the number of patients in the combination procedure group decreased to 64 (64 eyes) due to drop out, and mean IOP was 19.8±2.6 mm Hg. In addition, of these, 36 (36 eyes) had a Maklakov IOP of <22 mm Hg without the use of anti-glaucoma medications, 18 (28.1%) used topical anti-glaucoma medications, and 10 (15.6%) had a Maklakov IOP of >22 mm Hg with the use of anti-glaucoma medications. Moreover, mean number of hypotensive medications for achieving the target IOP was 1.1±0.3. At month 12, complete success of surgery was achieved in 57 patients (57 eyes; 72.1%), and partial success, in 13 patients (13 eyes; 16.5%). In addition, surgery failed in 9 patients (11.4%), and, of these, 4 patients (5.1%) underwent a repeat surgery. At month 24, complete success of surgery was achieved in 36 patients (36 eyes; 56.3%), and partial success, in 18 patients (18 eyes; 28.1%). In addition, surgery failed in 10 patients (15.6%), and, of these, 3 patients (4.6%) underwent a repeat surgery. As a result, 7 patients (7 eyes; 10.9%) of group 1 had a re-surgery within two years after initial surgery. In controls, mean IOP was 27.1±1.7 at baseline, decreased by 10.0±0.8 mm Hg (37%) to 17.1±0.5 mm Hg (р <0.001) at day 7, and practically did not change (17.9±0.5 mm Hg) at month 1 after surgery. At month 12, in the control group, the number of patients decreased to 68 (68 eyes) due to drop out, and mean IOP was 20.6±1.5 mm Hg. In addition, of these 68 (68 eyes), 14 (14 eyes; 20.5%) used topical prostaglandin analogs, 10 (10 eyes; 14.7%) had a Maklakov IOP of >22 mm Hg with the use of anti-glaucoma medications, and 5 (5 eyes; 7.4%) had a re-surgery. Moreover, mean number of hypotensive medications for achieving the target IOP was 1.5±0.8. At month 24, in the control group, the number of patients decreased to 55 (55 eyes) due to drop out, and mean IOP was 21.5±1.2 mm Hg (р <0.05). In addition, of these 55, 23 (23 eyes; 41.8%) had a Maklakov IOP of <22 mm Hg without the use of anti-glaucoma medications, and 20 (20 eyes; 36.4%) used topical hypotensive medications. Moreover, mean number of hypotensive medications for achieving the target IOP was 1.9±0.4. At month 12, complete success of surgery was achieved in 44 patients (44 eyes; 64.7%), and partial success, in 14 patients (14 eyes; 20.6%) of the control group. At month 24, complete success of surgery was achieved in 23 patients (23 eyes; 41.8%), and partial success, in 20 patients (20 eyes; 36.4%) of the control group. Five control patients (5 eyes; 7.4%) and seven control patients (7 eyes; 12.7%) had a re-surgery due to uncompensated IOP within a year and from month 12 to month 24 after surgery, respectively. Moreover, 3 control patients (3 eyes; 4.4%) and 3 control patients (3 eyes; 5.4%) had a surgery due to cataract progression within a year and from month 12 to month 24 after surgery, respectively. In the combination procedure group, mean MD before surgery and at month 12 and month 24 was -8.1 ± 6.9 dB, -8.5 ± 6.9 dB, and -8.9 ± 7.0 dB, respectively, with a mean annual decrease of 0.4±0.3 dB. In the control group, mean MD before surgery and at month 12 and month 24 was -8.8±7.6 dB, -9.2±7.6 dB and -9.7±760 dB, respectively, with a mean annual decrease of 0.5±0.3 dB. Mean PSD for group 1 was 5.4±4.2 dB before surgery, 6.1±4.2 dB at month 12 and 6.3±4.3 dB at month 24, with a mean annual increase of 0.2±0.2 dB. In addition, in eight patients (12.7%) of group 1, a decrease in PSD was of 0.2±0.1 dB. Mean PSD for the control group was 6.1±3.9 dB before surgery, 6.4±3.8 dB at month 12 and 6.8±3.9 dB at month 24, with a mean annual increase of 0.4±0.1 dB. Table 1 presents static automated perimetry (SAP) global indices before and after surgery for the combination procedure group and control group.
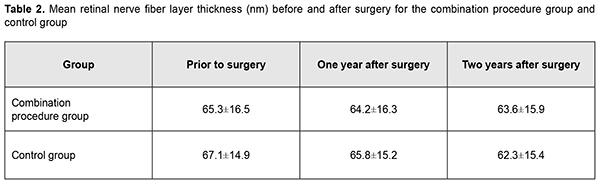
In patients of group 1, mean RNFL thickness was 65.3±16.5 nm before operation, and insubstantially decreased to 64.2±16.3 nm and 63.6±15.9 nm at month 12 and month 24, respectively. In patients of the control group, mean RNFL thickness was 67.1±14.9 nm before operation, and 65.8±15.2 nm and 62.3±15.4 nm at month 12 and month 24, respectively, but the difference was not significant. Preoperatively, vertical cup-to-disc ratio, neuroretinal rim area and neuroretinal rim volume were 0.67±0.21, 0.98±0.33 mm2, and 0.23±0.12 mm3, respectively, for the combination procedure group, and 0.62±0.14, 0.99±0.30 mm2, and 0.25±0.13 mm3, respectively, for the control group. Patients of both groups exhibited practically no change in morphometric characteristics of the optic disc at month 12 and month 24.
Discussion There are not many studies on the effect of surgical intervention on stabilization of glaucomatous neuropathy. Both functional and morphometric studies are utilized to assess the course of glaucomatous neuropathy. Although SAP is the gold standard of glaucoma monitoring, previous reports on the effect of a surgical IOP reduction on visual fields in the operated eyes have been contradictory. This may be partially explained by variability in methods used for comparison with respect to visual function progression, and variability in SAP data for a particular patient from one examination to another. Bhardwaj and colleagues [3] noted that studies varied with respect to characteristics utilized to assess progression in glaucomatous visual field defects and concluded that there is no perfect index for assessing progression in glaucomatous visual field defects. Mean deviation (MD) is, however, considered a more reliable measure than others. Pandey and Sujata [4] used MD and pattern standard deviation (PSD) to detect changes in visual field. Our findings for changes in visual fields demonstrate a direct relationship between the IOP reduction and the degree of glaucoma process stabilization. These findings are in agreement with those of the study by Bhardwaj and colleagues [3]. That study reported decreases in the slope of VF mean deviation (?1.0 ± 0.9 dB/year prior to surgery; ?0.2 ± 0.38 dB/ year postoperatively) and the slope of PSD (0.36 ± 1.01 dB/ year prior to surgery; 0.32 ± 0.34 dB/ year postoperatively) with a reduction in the average Goldmann IOP from 19 mm Hg to 13 mm Hg. Our findings of the positive effect of IOP reduction after the combined procedure of NPDS and diode laser trabeculoplasty on progression in glaucomatous visual field defects conform to those from the study by Koseki and co-authors [5] and Collaborative Initial Glaucoma Treatment Study (CIGTS). Koseki and co-authors [5] demonstrated a significant reduction in visual field progression rate after glaucoma surgery, whereas the CIGTS demonstrated that surgical treatment for glaucoma was more efficacious with regard to visual field preservation in patients with severe visual function loss. Changes in the optic disc precede and not always correlate with those in visual fields [6]. This indicates that both functional and morphometric studies are required to monitor the progression of glaucoma. That is why both these approaches to the assessment of disease progression were used for glaucomatous neuropathy monitoring in the current and numerous previous studies. OCT imaging of the optic disc and peripapillary retina is the most reliable, repeatable and objective monitoring method for POAG, and offers advantages like non-invasiveness, comfort for patient and objectivity. Normative databases incorporated into OCT instruments demonstrate the reliability, repeatability and reproducibility of this technique [7]. The results of our optic disc morphometric studies were less significant than OCT RNFL thickness studies. Pandey and Sujata [4] used changes in mean average RNFL thickness and average RNFL thickness measurements in temporal, superior, nasal, and inferior quadrants to assess the progression of glaucoma. In the current study, at month 12 and month 24 after surgery, mean IOP was 19.1±1.2 mm Hg and 19.8±2.6 mm Hg, respectively, and RNFL thickness decreased by 1.1 nm/year (from 65.3 ± 16.5 nm to 64.2±16.3 nm) and by 0.6 nm/year (to 63.6±15.9 nm), respectively. These results were close to those of Diniz-Filho and colleagues [8], who found that, with a reduction in Goldmann IOP to 15 mm Hg (i.e., Maklakov IOP of approximately 20 mm Hg) and 25 mm Hg (i.e., Maklakov IOP of approximately 30 mm Hg), RNFL thickness decreased by 0.82 nm/year and 2.82 nm/year, respectively. The mechanism of hypotensive action of NPDS with a simultaneous laser trabeculoplasty ab externo is associated with activation of trabecular tissue that offers the major proportion of the resistance to aqueous outflow in POAG. Compared to NPDS alone, this combination procedure offers a more apparent and stable hypotensive action both at the early and late follow-up. Thus, at month 12 and month 24 after surgery, complete or partial success was achieved in 88.6% of patients of group 1 versus 85.3% of the control patients, and 84.4% of patients of group 1 versus 78.2% of the control patients, respectively. Correspondingly, compared to the controls, patients treated with the combination procedure exhibited better glaucoma stabilization based on SAP data and results of morphometric studies. Conclusion First, this study showed a more profound hypotensive effect in glaucoma patients treated with a combination procedure of NPDS with micropulse laser ab externo in comparison to NPDS alone. Second, after surgery, progression of glaucomatous optic neuropathy was slower in the combination procedure group than in the control group, with mean decrease in MD and mean increase in PSD of 0.4±0.3 dB and 0.2±0.1 dB, respectively, in the former group, versus 0.5±0.3 dB and 0.4±0.1 dB, respectively, in the latter. Finally, in the combination procedure group, there was practically no change in morphometric optic disc parameters or RNFL thickness during a two-year follow-up after surgery, which is evidence of stabilization of glaucoma. References 1.Hodapp E, Parrish RK, Anderson DR. Clinical decisions in glaucoma. St. Louis: The CV Mosby Co; 1993. pp. 52–61. 2.Rudavska LM. [Clinical efficacy of the combined non-penetrating deep sclerectomy with the simultaneous diod laser trabeculoplasty ab externo in patients with primary open-angle glaucoma. Long-term results]. Oftalmokhirurgiia. 2016;2:35–40. Russian. 3.Bhardwaj N, Niles PI, Greenfield DS. The impact of surgical intraocular pressure reduction on visual function using various criteria to define visual field progression. J Glaucoma. 2013;22:632–7. 4.Pandey AN, Sujata S. Study of long term structural and functional changes in medically controlled glaucoma. Int J Opthalmol. 2014;7:128-32. 5.Koseki N, Araie M, Shirato S, Yamamoto S. Effect of trabeculectomy on visual field performance in central 30 degrees field in progressive normal-tension glaucoma. Ophthalmology. 1997 Feb;104(2):197-201. 6.Xin D, Greenstein VC, Ritch R, et al. A comparison of Functional and Structural measures for identifying progression of glaucoma. Invest Ophthalmol Vis Sci. 2011 Jan 25;52(1):519-26. 7.Kotowski J, Wollstein G, Folio L, et al. Clinical use of OCT in assessing glaucoma progression. Ophthalmic Surg Lasers Imaging. 2011 Jul;42 Suppl:S6-S14. 8.Diniz-Filho A, Abe RY, Zangwill LM. Association between Intraocular Pressure and Rates of Retinal Nerve Fiber Layer Loss Measured by Optical Coherence Tomography. Ophthalmology. 2016; 123: 2058-65.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|