J.ophthalmol.(Ukraine).2017;6:72-77.
|
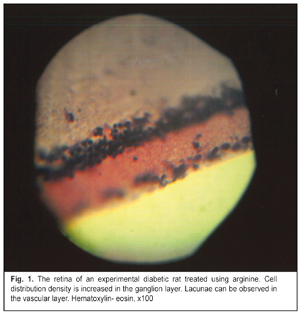
https://doi.org/10.31288/oftalmolzh201767277 Metabolic correction of experimental diabetic retinopathy I.V. Savitskyi1, Prof., Dr. Sc. (Med)1, V.V. Semenko2 , V.M. Serdiuk2,3 1Odessa National Medical University 2Dnipropetrovsk Regional Ophthalmological Clinical Hospital DOKOL 3Dnipropetrovsk Medical Academy, Ministry of Health of Ukraine Odessa, Dnipropetrovsk, Ukraine E-mail: farmakod@ukr.net Introduction. Diabetic retinopathy (DR) is diagnosed in 50% of the total number of patients suffering from type 1 diabetes mellitus with diabetes duration of over 10 years and in 75-90% of patients with diabetes duration over 15 years. Purpose. To ground the possibility of using arginine for correction of vascular changes in the eye at early stage of DR. Materials and Methods. The study involved outbred white Wistar rats divided into three groups: Group 1, unexposed animals served as controls (Control group); Group 2, animals with a diabetes mellitus model (DM group); Group 3, diabetic animals which received 7% arginine solution (DM+arginine group). Results. Glucose test revealed no significant difference between Group 1 and Group 3 (р=0.14) while the difference was highly significant between Groups 2 and 3 (р=0.0019). Histological study of the eyeball tissues showed that fibers were not combined into the homogeneous mass, separate fibers could be defined. In the photoreceptor layer, structural elements were pale stained but had high density of distribution. The outer granular layer of the retina was characterized by nuclei with rather dense distribution to each other. The nuclei were relatively large, round, and brightly stained. The inner granular layer corresponded to the description of the outer granular layer. In the ganglion cell layer, neuron nuclei were round, well-stained and distributed rather evenly. Conclusions. Arginine enabled to preserve the structure and functions of vessels and to reduce the disease severity. Keywords: diabetes mellitus, diabetic retinopathy, arginine, histological examination, eyeball, NOS. Background Diabetic retinopathy (DR) is diagnosed in 50 % of patients suffering from type 1 diabetes mellitus with diabetes duration of 10-15 years and in 75-90% of patients with diabetes duration over 15 years [1]. Correction of vascular pathology is one of the most important directions of DR treatment. One of key points of vascular disorders in diabetes mellitus (DM) is endothelial dysfunction [2]. A number of clinical and experimental studies have reported that endothelium dysfunction and disorders of elastic properties of arteries are related to risk of vascular diabetic complications [3, 4, 5, 6]. Endothelium dysfunction (ED) is caused by altered synthesis of nitric oxide (NO) which plays an important physiological part due to a wide range of bioregulatory effects [7, 8]. NO in vascular homeostasis support is characterized by vascular tone regulation, proliferation and apoptosis, regulation of oxidation processes, and angioprotective properties. No is also a strong peripheral vasodilator [8]. The main substrate for NO synthesis is arginine [9]. Arginine has a quite wide range of important functions but its main role is that it is a substrate for nitric oxide synthesis [10-14]. So, it is arginine that is required for correction of endothelial dysfunction, due to DM particularly, since the endothelium can synthesize necessary substrates from this amino acid [15]. Purpose. To ground the possibility of using arginine for correction of vascular changes in the eye at early stage of DR. Material and Methods The study involved outbred white Wistar rats, weighted 180 to 200 g. The animals were divided into three groups: Group 1: 20 unexposed animals served as controls (Control group); Group 2: 30 animals with a diabetes mellitus model (DM group); Group 3: 30 animals with a diabetes mellitus model which received 7% arginine solution (DM+arginine group). DM was modeled using a three-time abdominal alloxan injection in a dose of 7.5 ml with a five day interval. Alloxan was administered on the background of the animals free drinking a 5% fructose solution. The experiment lasted 30 days. This model was characterized by 100% survival of the experimental animals. The animals were euthanized by decapitation under brief ether anesthesia following Regulations for Studies Involving Experimental Animals approved by Order No249 of the Ministry of Health of Ukraine from 3 March 2012 and Ukraine’s Act No3447-IV On the Protection of Animals against Cruelty (with changes dated 15 December 2009 and 16 October 2012). The animals were taken a specimen of blood (5 ml) and glucose test in the blood serum was performed using a semi-automatic biochemical analyzer (Microlab-300, Netherland) with a glucose oxidase test. Then enucleation was performed. One eyeball was fixed in 4% paraformaldehyde for 24 hours; afterwards, the obtained samples were passed through a series of alcohol solutions of increasing concentration and poured into celloidin according to a common method. Histologic sections of 7-9 microns were prepared from the obtained blocks and stained with hematoxylin-eosin. A light microscope was used to examine the obtained samples and to detect structural changes in the formations of the eyeball. Another eyeball was frozen in liquid nitrogen (-196 °С) and the obtained blocks were cut to prepare cryostat sections, 11 µm thick, which were histologically tested to study the activity of nitric oxide synthase (NOS). Here, we used the Lloyd’s method based on using a unified solution to which the solutions of a corresponding substrate and coenzyme are added [16]. The NOS activity was assessed according to the color of granules and the background as well as to the comparative granule distribution density: • grey, greyish-yellow staining of small formazan granules gives the evidence of NOS trace activity in the cell body; grayish or grayish-purple staining of the background gives the evidence of trace NOS activity. • grey-yellowish staining of small and medium granules in the cells and grayish-yellowish staining of the background indicate weak NOS activity • yellow, yellow-brown staining of small and medium granules in the cells on the yellowish or sand-colored background is considered as moderate NOS activity. • yellow-brown or brown staining of large or medium granules with yellowish background staining is an evidence of high NOS activity. Seeing that the series of the measured index in all the samples did not differ from the normal statistical distribution, which was tested using the Shapiro-Wilk test which is most sensitive to relatively small sample sizes, we used descriptive statistics (mean ± standard error) for further mathematically statistical data processing; intergroup comparisons were performed using Student's criterion. Studies of the rats receiving arginine against the background of experimental DM were compared with the results of the group with an aloxane DM model that we performed and reported in 2017 [17]. Results Results of biochemical studies, descriptive statistics and statistical significance of intergroup comparison are given in Table 1. Data obtained show that the greatest difference was noted between Control group and DM group. Highly significant difference was also noted between DM group and DM+argenine group. Experiment revealed no significant difference between DM+argenine group and Control group, which confirmed a corrective action of this amino acid in diabetic retinopathy. Histological study of the eyeball tissues (Fig. 1) draw attention to a swelling of protein coat tissues but the fibers were not combined into the homogeneous loose mass, separate fibers could be defined, which was common for Control group. Medium and large granules were disposed evenly in one mass and compactly packed within the pigmented layer. In the photoreceptor layer, structural elements were pale stained but had high density of distribution. The outer granular layer of the retina was characterized by nuclei with rather dense distribution to each other. The nuclei were relatively large, round, and brightly stained. The inner granular layer corresponded to the description of the outer granular layer, which is typical of norm. In the ganglion cell layer, neuron nuclei were round, well-stained and distributed rather evenly; no swelling and distribution sparsity was observed. In the stromal layer of the choroid were defined single lacunae and vessels with vibrantly stained endothelial cells. The lens capsule was lined with a single layer of evenly distributed cells; the cell nuclei were small and thoroughly stained. In the lens not far from periphery, there were medium stripes with nucleus fragments and short pale-stained eosinophilic fibers.
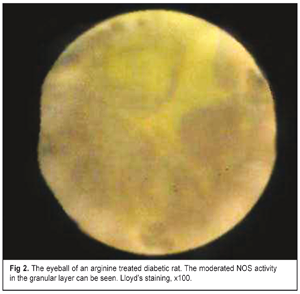
Figure 2 demonstrates data on histochemical study of the NOS activity in the eyeball tissues of the experimental rats in Group 3. The stromal layer of the choroid was characterized by grey-yellow staining of the background and medium-sized yellow granules on the edges of single lacunae. In the granular layers, background had grey-yellow staining. In the ganglion cell layer there were diffused small gray-yellow medium-sized granules.
In DM Group without argenine treatment, the NOS activity study revealed that the stromal layer of the choroid had grey-yellowish staining and there were single grey-black granules along the outline of the vascular lacunae. In the granular layers, staining was pale-grey and grey-purple with single grey-yelowish and grey-black granules. In the ganglion cell layer, there were single cells with grey and grey-black granules in the cytoplasm. In Control group, red blood cells in the vascular lacunae were yellow-brown. Small yellow and grey-black granules on the edge of the lacunae were unevenly distributed. In the granular and ganglion cell layers, the background was grey-yellow; cells with a moderate quantity of grey and grey-yellow granules could be seen. The background of the choroid was grayish-yellowish-brown. Discussion Based on hismochemical study we can speak of the close-to-norm moderate activity of NOS in the retinal layers of the DM rats having been treated by arginine while the NOS activity was weak in the experimental animals which had not received arginine for simulated DM [17]. Our previous papers have reported that simulated diabetes mellitus leads to the increased blood glucose level and the decreased NOS activity. Also, histological analyses of the eyeball tissues revealed changes as follow: a swelling of protein coating tissues, fibrous fibers combined in the homogeneous loose bulk without separate fibers; granules in the pigment layer were large and spread within the whole area; structural elements in the photoreceptor layer had pale staining and insignificant distribution density; in the outer granular retinal layer, separate nuclei could be seen, which can testify to their sparse distribution. The nuclei were pathologically increased and of pale staining. In the inner granular layer there was an increased quantity of nuclei which were not identical to those in the outer layer. In the ganglion layer, neuron nuclei were unevenly distributed and swollen. In the stromal layer, we revealed the absence of lacunae as well as a swelling and disordered distribution of endothelial cells. Also, in the DM only group, we draw attention to the fact that in the anterior capsule lens epithelium there were nucleus fragments in the lens and an increased quantity of nuclei spread along the lens periphery. Besides, the cell nuclei located on the lens capsule are of a medium size and of moderate staining [17]. In the present paper, the above results indicate that the histological picture of the eyeball differed from the results in the group with untreated experimental diabetes and was close to those in the Control group. This points at the fact that arginine treatment for DR is successful and clinically significant. We developed a MD model based on alloxan administration into the experimental animals. The closest to our model is the model in which DM is induced as follows: 1) For a subcompensated DM model, rats were administered alloxan intraperitoneally after overnight fast; alloxan administration was repeated sequentially in a dose of 5 mg/100g, 7 mg/100g, and 5 mg/100g of the body weight with a seven day interval. 2) For an uncompensated DM model, 5 mg/100g body weight of alloxan was administered three times every other day [18]. The alloxan DM model to be spread is imbedded due to a high toxic level of alloxan, which was determined for previously used alloxan models [19, 20]. As for nephrotoxicity, alloxan administration in previously known doses resulted in necrosis of certain sections of the convoluted renal tubules and, consequently, in a renal disease that develops in some animals before DM and can be a cause of death of the rats within the first five days after alloxan administration [19, 21]. However, this is the alloxan DM model that is the most appropriate one to assess the efficacy of the developed treatment due to a mechanism of the alloxan effect on beta cells. This is generation of alloxan of reactive oxygen species in the cyclic reaction with hyaluronic acid. These reactive species are responsible for the death of the beta cells which have a low antioxidative defence capacity [22, 23]. We calculated the optimal dose of alloxan to be administered which did not cause the death of animals (there was no fatal cases in our experiment) and was an effective model not only for DM in general but also for diabetic retinopathy, which was evidenced by the results of our experiment. The preferred alloxan dose makes it possible to obtain less severe and more physiological changes which are common for early DM stages. Another peculiarity of our model is that the experimental animals were administered fructose solution which is an additional load to carbohydrate metabolism and which disintegrate the normal metabolic process. The mechanism of the protective action of arginine on the vessels in diabetes can be explained as follows. L-arginine (?-amino - ?- guanidino-valeric acid), a precursor of L-ornithine, L-citrulline, L-glutathione, gamma-aminobutyric acid, spermitin and other compounds, is one of the most polarized, positively charged amino acids [24, 25]. It was first isolated by E. Schulze and E. Steiger in1886 and its structure was defined by E. Schulze and E. Winterstein in 1897. It contains a positively charged Rh-group and is a component of basic proteins in the body [26]. In the body, L-arginine is metabolized in two alternative ways which can run simultaneously: 1) oxidizing (with NOS involved) with the formation of L-citrulline and NO; 2) non-oxidizing (with arginase I involved) with the formation of L-ornithine and urea [27]. In the biological systems, L-arginine plays an important role in the synthesis of a number of anabolic hormones, polyamines, and nitric oxide [25]. L-arginine, getting with the food into the body, is absorbed at the small bowel and is transported into the liver where the main quantity of arginine is utilized in the ornithine cycle and the rest part, unmetabolized in the liver, becomes a substrate for NO production [28]. L-arginine selectively improves the endothelial function when L-arginine concentration in the plasma is law; it can cause direct vasodilatation when its concentration is medium; and L-arginine causes unspecific vasodilatation when its concentration is high [8, 13, 28, 29]. It is also known that L-arginine can potentiate synthesis of nitric oxide and reduce macular edema due to the restored activity of endothelial nitric oxide synthase (eNO-S) [13]. L-arginine prevents oxidation of BH4 (tetrahydrobiopterin) which is the main NOS cofactor. Braking the oxidation of low-density lipoproteins (LDLP) which, in turn, reduce the NO level, L-arginine breaks eNO-S and caveolin complex potentiated by oxidized LDLP, which suppresses the NOS activity [29, 30]. Also, this amino acid support the NO action through increasing the biological activity of NO by the direct antioxidant activity, decreasing the noradrenaline activity and stimulating the histamine production from main cells, which supplements the vasodilatory action [30]. In vivo using L-arginine decreases the level of NOS-mediated superoxide [31]. L-arginine helps overcome the blockade of eNOS expression which is caused by NG-monomethyl-L-arginine and asymmetric dimethyl arginine (endogenous eNOS inhibitors) as well as the increased activity of arginase in atherosclerosis [32, 33, 34, 35]. L-arginine decreases the concentration of endothelin-1 which is a vasoconstrictor and an important modulator of endothelial dysfunction [34, 35, 36]. A pathway L-arginine – NO, which has a direct effect on the state of the endothelium, provides the optimal functioning of the cardiovascular system [37, 38, 39], improves the endothelial function against the background of hypercholesteremia [40, 41]. It is important for balancing the inflammatory response of the body [42], apoptosis [43], and protection from oxidative damage [36]. L-arginine increases atrial cGMP levels and, thus, enhances a vagal effect and inhibits a sympathetic component [36]. The effect of L-arginine on the systemic circulation and microcirculation in infuse therapy for experimental hemorrhagic shock improves the work of the heart muscle and promotes the survival of animals [44]. The efficacy of L-arginine correction for the endothelial state in patients with obliterating atherosclerosis is characterized also by the prolonged action of this amino acid. A positive clinical effect also appears in increasing the distance that a patient can pass without pain [45]. Conclusions 1. The experimental alloxan DM model with a fructose solution we used turned out to be effective in terms of disease stimulation and was characterized by 100% survival of the experimental animals. 2. Arginine treatment for simulated DM is beneficial in preserving the structure of the vessels and, as a consequence, the structure of the eyeball layers and contributed to reducing the severity of the pathological process.
3. The NOS activity increased against the background of arginine treatment, as compared with the untreated experimental diabetic animals, and was close to the normal range, which gives the evidence of improved functioning of the endothelium. References
|