|
https://doi.org/10.31288/oftalmolzh20166611
Effect of tear substitutes with various sodium hyaluronate levels on the condition of eye anterior segment in dry eye syndrome patients
G.I. Drozhzhina, Dr. Sc. (Med.), Prof.
T.B. Gaidamaka, Dr. Sc. (Med.)
L.F. Troichenko, Cand. Sc. (Med.)
Filatov Institute of Eye Diseases and Tissue Therapy, NAMS of Ukraine
Odessa, Ukraine
E-mail: cornea@te.net.ua
The purpose of the present paper was to compare the effect of tear substitutes with various sodium hyaluronate levels (0.21% and 0.4%) on eye anterior segment condition and to determine the informative value of various diagnostic tests in dry eye syndrome patients
Materials and methods We followed up 40 patients (80 eyes), aged (57.8±7.5SD), with moderate dry eye syndrome (DES). All the patients were divided into two groups: group I included 20 patients (40 eyes) receiving 0.21% Optinol eye drops; group II included 20 patients (40 eyes) receiving 0.4% Optinol eye drops. Ophthalmic examination included Ocular Surface Disease Index (OSDI), biomicroscopy of the anterior chamber, fluorescence microscopy, tear break-up time for determining tear film stability, Schirmer’s I test and Jones' Schirmer II test to measure total and basal tear production, respectively; and best corrected visual acuity measurement.
Results The use of hyaluronic acid-based tear substitute, 0.21% and 0.4% Optinol, showed good therapeutic efficacy and drug tolerability. The use of 0.21% and 0.4% Optinol eye drops significantly increased subjective complaints of DES patients, which is evidenced by an OSDI index. For diagnostics of condition of the anterior eye segment in DES, it is most appropriate to use Schirmer II test, TBUT and LWE assessment which are most sensitive both in DES diagnostics and assessment of treatment quality. Since the 0.4% Optinol eye drops have a more pronounced moistening, anti-inflammatory, and protective action, it is reasonable to use them in the treatment of dry eye syndrome which is accompanied with apparent ocular surface tissue inflammation.
Key words: dry eye syndrome, 0.21% and 0.4% sodium hyaluronate
Background
Dry eye syndrome (DES) is one of the most common diseases which is observed in 9-18% of adults in developed countries and in more than 67% cases of persons over 60 y/o. In 2007, International Dry Eye Work Shop defined dry eye syndrome as “a multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability with potential damage to the ocular surface. It is accompanied by increased osmolarity of the tear film and inflammation of the ocular surface” [15, 19].
Over last thirty years, DES detection rate increased 4.5 times [3, 19]. Therefore, there have been numerous papers concerned with studying DES etiology and pathogenesis as well as developing the drugs to substitute differentially a certain layer of the tear film [4, 6, 11, 16]. For artificial tear drugs are used, as a rule, many times during the day and for a long time, and quite often during the whole life, the development of up-to-date artificial tears follows the search for natural molecules and compounds enabling to integrate into the tear film and having protective properties for the corneal epithelium and conjunctiva.
One of these compounds is hyaluronic acid. A unique property of this natural bioinert polysaccharide has opened the door for its wide use not only as a component of dispersive viscoelastic in cataract and glaucoma surgeries but also as a main component of tear substitute drugs.
Proteoglycans are high-molecular carbohydrate-protein compounds that comprise the main substance of the extracellular matrix and are known to be very important chemical components of connective-tissue structures in eye tissues including the cornea. A carbohydrate component of proteoglycans is glycosaminoglycans [1, 5]. One of the main biopolymers of extracellular substance of the connective tissue is hyaluronic acid (HA), more precisely, sodium hyaluronate or hyaluronan, which determines, in many regards, functional properties of tissues and plays a stabilizing and protector role [7,12].
Hyaluronic acid is a crucial component of extracellular matrix in the organism and can be found in many body fluids including the saliva and tears. HA has unique viscoelastic and hygroscopic properties and is able to keep the water volume 1 000 times exceeding its own volume. Due to these properties, it can be used for long-term and intensive ocular surface lubrication in DES. The epithelium of the cornea and conjunctiva is known to express receptors to HA. HA is shown to take a significant part in cell proliferation and migration on the ocular surface [10].
Intra-articularly-injectied hyaluronic acid has been found to have anti-inflammatory and chondroprotective action in experimental arthritis, which has been confirmed by studying the content and level of glycosaminoglycans in synovial system of the damaged joint including the articular cartilage [2].
Sodium hyaluronate (SH) is the sodium salt of hyaluronic acid obtained biotechnologically. It is white powder containing no less that 90% of SH, molecular weight not less than 1.05 х 106 Da. The powder is highly-soluble in water and formed into viscous uncolored slightly-opalescent gel, рН 6.0-7.5 (0.1% solution). Regarding its action, SH is similar in every way to hyaluronic acid; it facilitates tissue regeneration and can stimulate cell migration and proliferation.
Sodium hyaluronate drugs are one of the most common tear substitutes using in DES.
When developing artificial tears, a special attention is drawn to preservative-free forms. Studies on the use of benzalkonium chloride-preserved drugs, including even little concentration (0.02-0.05%), have evidenced the presence of side effects of this preservative. It has been found that a significant part of benzalkonium chloride (BAK) is accumulated in the corneal and conjunctival epithelium and in the stroma, and less in the iris, lens, choroid, and retina [8, 9].
In this respect, preservative-free tear substitutes are preferable to date. One of such medication is Optinol, benzalkonium chloride eye drops, Jadran-Galenski Laboratorij d.d. (Croatia). Optinol contains benzalkonium chloride in concentration 21.15 mg or 40.28 mg; it is preservative-free with sodium chloride, anhydrous sodium phosphate, sodium dihydrogen phosphate, dehydrates, and purified water as inactive ingredients.
Since DES is a common disease and there are a quite great number of tests to diagnose DES, it is important to study the informative value of various functional tests in DES.
The purpose of the present paper was to compare the effect of tear substitutes with various sodium hyaluronate levels (0.21% and 0.4%) on the condition of eye anterior segment and to determine the informative value of various diagnostic tests in dry eye syndrome patients.
Materials and Methods
The study was conducted at Corneal Pathology Department at Filatov Institute of Eye Diseases and Tissue Therapy. We followed up 40 patients (80 eyes), aged (57.8±7.5SD), with moderate dry eye syndrome (DES). There were 12 men and 28 women. All the patients were divided into two groups: group I included 20 patients (40 eyes) receiving 0.21% Optinol eye drops; group II included 20 patients (40 eyes) receiving 0.4% Optinol eye drops. In both groups, Optinol was prescribed to instill 1-2 drops 4 times a day for four weeks. The groups compared did not differ regarding DES symptoms' severity, sex, or age. The treatment was not combined with any other tear substitutes. Apart from Optinal eye drop, all patients were instilled 2% boric acid antiseptic. Exclusion criteria were the presence of eyelid position abnormalities, lagophthalmos, uncompensated diabetes mellitus, and rheumatoid arthritis.
Ophthalmic examination included Ocular Surface Disease Index (OSDI), biomicroscopy of the anterior chamber, fluorescence microscopy; tear film stability was determined using tear break-up time; total tear production was measured using Schirmer’s I test; Jones' Schirmer II test was used to measure basal tear production; and best corrected visual acuity was measured.
To assess Ocular Surface Disease Index, each patient completed a standard OSDI questionnaire [13]. OSDI is graded according to answers to 12 questions about the eye’s discomfort: light sensitivity, grittiness, painful and sore eyes, blurry vision; limitation in reading, driving at night, working with a computer; feeling uncomfortable in windy conditions, in places or areas with low humidity, in areas that are air conditioned. Each symptom is valued from 0 to 4 scores. Sum of scores is from 0 to 100; the higher is the score, the more severe DES is.
Tear break-up time (TBUT) was assessed as follows: a patient was asked to look down and, after pulling the upper eyelid, the cornea was fluorescein stained using 0.2% fluorescein sodium. Keeping time with stop watch, the stained tear film was observed under the slit lamp until the appearance of a break which looks like a black “hole” or gap. The watch was stopped at the moment when the hole started to increase in size or to branch radially. A TBUT of 10-20 seconds is considered normal [3].
Total tear production was assessed using Schirmer’s I test [18]. The end of a filter paper strip (~5 mm) was bent at 40-45° and placed underneath the lower eyelid in the lateral third of the eye fissure. Herewith, the bent end of the strip reached the bottom of the inferior conjunctival fornix (not touching the cornea) and the bend touched the lid margin. Both eyes were tested at the same time. The patient was asked to close the eye; after 5 minutes, the strips were removed and the amount of moisture on each strip was measured. Normally, not less than 15 mm of each paper strip are moistened for 5 minutes.
Jones' Schirmer II test was used to measure basal tear production (L. T. Jones, 1966). The difference from Schirmer I test consists in preliminary anesthesia of the ocular surface, which enables to exclude reflex tear production. 10 mm moistening of a standard test strip is considered to be normal [14].
Biomicroscopy of the anterior chamber of the eye was used to detect the presence of the Lid Parallel Conjunctival Folds (LIPCOF) and the degree of Lid Wiper Epitheliopathy (LWE).
An overall result of LIPCOF (LIPCOF grades) is scored by summing up the nasal and temporal LIPCOF results: grade 0: no permanently present folds; grade 2: one permanently present fold; grade 3: two permanently present folds (<0.2 mm in norm); grade 3: three and more permanently present folds (>0.2 mm in norm) [17]. Folds in DES are caused by looseness of the conjunctiva, breakdown of elastic fibers, aging and mechanic interaction between the lower lid and the conjunctiva.
LWE was assessed according to a degree of fluorescein corneal staining in diffuse slit lamp lightening after blue filter fluorescein instillation (%) and graded as follows: grade 0: horizontal length of staining < 2 mm, < 25% of width of wiper area; grade 1: horizontal length of staining 2-4 mm, 25-50 % of width of wiper area; grade 2: horizontal length of staining 5-9 mm, 50-75% of width of wiper area; grade 3: horizontal length of staining >9 mm, >75% of width of wiper area [20]. LWE in DES occurs in a result of friction between lids and the ocular surface.
We compared treatment outcomes between the two groups (I and II) (p) as well as pre- and post-treatment parameters in the groups I and II, (р1) and (р2), respectively.
Student criterion (t) was used for comparison of variables. A P-value less than 0.05 was considered to be statistically significant. Statistical data processing was performed using STATISTICA 7 software.
Results and Discussion
At baseline the main complaints in both groups were foreign body sensation, sandy feeling in 90% of patients, dryness and burning in 85%, poor wind tolerability, discomfort when working on a computer in 75%, and light sensitivity, blurry vision in 65% of patients
OSDI at baseline (pre-treatment) was (62.8±26.4SD) and (60.6±24.4SD) scores, p>0.05, in group I and II, respectively. After treatment, 80% of group I and 85% of group II patients noted subjective improvement. OSDI decreased and was equal to (42.6±20.4SD) and (40.8±22.4SD), (р= 0.8, р1= 0.01, р2= 0.01), in groups I and II, respectively. (Table 1).
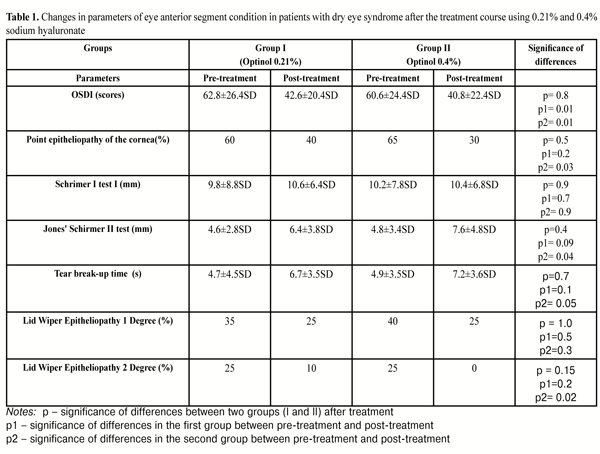
At baseline, corneal fluorescein staining revealed point epitheliopathy in 12 (60%) and 13 (65%) patients in group I and II, respectively. After treatment, point fluorescein staining was noted in 8 (40%) and 6 (30%) patients, (р= 0.5, р1=0.2, р2= 0.03), in groups I and II, respectively. The fact that corneal epithelium regeneration is more apparent in instillations with higher sodium hyaluronate level can be explained by SH anti-inflammatory properties.
Schirmer I test showed that total tear production at baseline averaged (9.8±8.8SD) mm and (10.2±7.8SD) mm, in group I and II, respectively. Treatment performed resulted in total tear production equaling to (10.6±6,4SD) and (10.4±6.8SD) mm, (р=0.9, р1=0.7, р2= 0.9), in groups I and II, respectively. High quantity of tear production in patients is a result of an increased reflex part of the tears due to test-strip irritation of the eye.
Jones' Schirmer II test showed that basal tear production index at baseline was (4.6±2.8SD) mm and (4.8±3.4SD) mm, in groups I and II, respectively. Post-treatment basal tear production index was (6.4±3.8SD) and (7.6±4.8SD) in groups I and II, respectively, (р=0.4, р1= 0.09, р2= 0.04).
The difference between total and basal tear production defines reflex tear production which at baseline was (5.2±6.0SD) mm and (5.4±4.4SD) mm in groups I and II, respectively; and, after treatment, it was equal to (4.2±2.6SD) mm and (2.8±2.0SD), in groups I and II, respectively, (р=0.06, р1=0.5, р2=0.047).
Thus, on treatment completion, basal tear production was apparently increased and reflex tear production was decreased under the influence of hyaluronate of higher concentration, 0.4%, which is supposed to be connected with a more apparent moistening, protective, and anti-inflammatory action of the drug.
Tear break-up time (TBUT) before using 0.21% and 0.4% Optinol was (4.7±4.5SD) s and (4.9±3.5SD) s, respectively. After treatment, TBUT was equal to (6.7±3.5SD) s and (7.2±3.6SD) s, in groups I and II, respectively, (р=0,7, р1=0,1, р2= 0,05 ).
Lid Parallel Conjunctival Folds were noted in 10 (50%) and 11 (55%) patients in groups I and II, respectively. LPCF index did not change after treatment.
Lid Wiper Epitheliopathy grade I was noted at baseline in 7 (35%) and 8 (40%) patients of groups I and II, respectively. After treatment, LWE grade I was noted in 5 (25%) patients of each group, (р = 1.0, р1=0.5, р2=0.3).
During the treatment course, no allergic reaction was noted in patients of both groups.
Group II patients receiving 0.4% Optinol had shot-term blurring upon drop instillation in four cases (20%) (p=0.04). Due to moistening, protective, and anti-inflammatory properties of 0.4% Optinol, it can be used in combination with 0.21% Optinol.
Conclusions
1. The use of hyaluronic acid-based tear substitute, 0.21% and 0.4% Optinol, showed good therapeutic efficacy and drug tolerability.
2. The use of 0.21% and 0.4% Optinol eye drops sighificantly increased subjective complaints of DES patients, which is evidenced by a decreased ocular surface disease index.
3. To assess the anterior eye segment condition in DES, it is most appropriate to use Schirmer II test, TBUT and LWE assessment which are most sensitive both in DES diagnostics and assessment of treatment quality.
4. Since 0.4% Optinol eye drops have a more pronounced moistening, anti-inflammatory, and protective action, it is reasonable to use them in the treatment of dry eye syndrome which is accompanied with apparent ocular surface tissue inflammation.
References
1. Berezov TT, Korovkin BF. [Biological chemistry]. M.: Meditsina; 2002.704p. Russian.
2. Blinnikova VV. [Pathogenic rational for local therapy of experimental adjuvant arthritis using sodium hyaluronate]. Author’s thesis for Cand. Sc. (Med). Saratov; 2006. 18p. Russian.
3. Brzheskii VV, Somov EE. [Corneal conjunctival xerosis (diagnostics, clinic, treatment)]. Saint Petersburg: Saga; 2002. 142p. Russian.
4. Maichuk IuF, Iani EV. [Drug of a new pathogenic action in dry eye therapy]. Kataraktalnaia i refraktsionnaia khirurgiia. 2011;11(2):2-7. Russian.
5. Pavlova VN, Pavlov GG. [Articular cartilage and synovial membrane: specific of interaction in norm and in disease. [Local therapy in rheumatic disorders: Proceedings of All-Union conference. M.: 1988; 111-2. Russian.
6. Somov EE. [Ethiopathogenetic basis of dry eye syndrome and treatment approach principles]. Proceedings of jubilee scientific practical conference dedicated to 75 anniversary of the first pediatric ophthalmology department in Russia. Saint Petersburg. 1-16 October 2010. 482-8. Russian.
7. Abatangelo G. Hyaluronan: biological role and function in articular joint / G.Abatangelo, M.O'Regan // Eur. J. Rheum. Inflam. – 1995. – V.15. – P.9-16.
8. Ammar DA, Noecker RJ, Kahook MY. Effects of benzalkonium chloride-preserved, polyquad-preserved, and sofZia-preserved topical glaucoma medications on human ocular epithelial cells. Adv. Ther. 2010;27:1-9.
Crossref
9. Ayaki M, Yaguchi S, Iwasawa A. Cytotoxicity of ophthalmic solution with and without preservatives to human corneal endothelial cells, epithelial cells and conjunctival epithelial cells. Clin. Exp. Ophthalmol. 2008;36:553-9
Crossref Pubmed
10. Bucher F, Bachmann B, Bock F, Gross D, Cursiefen C, Kruse FE. Impact of hyaluronic acid, panthenol and its combination on epithelial wound healing in murine corneas. Investigative Ophthalmology & Visual Science. 2009;4(50):6285.
11. Bischoff G, Khaireddin R. Lipidsubstitution bei kontaktlinsenassoziiertem Trocken Auge. Aktuelle Kontaktologie. Sept. 2011:1-4.
12. Carson SE, Wolf J. Interaction between synoviocytes and extracellular matrix in vitro. Ann. Rheum. Dis. 1995; 54:413-6.
Crossref Pubmed
13. Jones LT. The lacrimal secretory system and its treatment. Amer. J. Ophthalmol. 1966;62(1):47-60.
Crossref Pubmed
14. Mishima S, Kubota Z, Farris RL. The tear flow dynamics in normal and in keratoconjunctivitis sicca cases. In: Solanes M. P., editor. Ophthalmology; Proceedings of the XXI International Congress; Mexico, DF. 8–14 March, 1970; Amsterdam: Excerpta Medica; 1971. 1801–5.
15. Nichols K, Foulks G, Bron A. The International Workshop on Meibomian Gland dysfunction. Invest. Opthalmol. and Vis. Sci. 2011;52:1917-29.
Crossref Pubmed
16. Dougberty BE, Nichols JJ, Nicbols KK. Ocular Surface Disease. Rasch Analysis of the Ocular Surface Disease. Invest. Ophthalmol. Vis. Sci. 2011;52(12):8630-35.
17. Pult H, Riede-Pult BH, Murphy PJ. The relation between blinking and conjunctival folds and dry eye symptoms. Optom Vis Sci. 2013;90(10):1034-9.
18. Schirmer O. Studie zur Physiologie und Pathologie der Tranenabsonderung und Tranenabfuhr. Graefes Arch. Ophthalmol. 1903;56(2):197-291.
19. The international Dry Eye Workshop (DEWS). The Ocular Surface 2007;5:65-90.
20. Varikooty J, Srinivasan S, Subbaraman L, Woods CA, Fonn D, Simpson TL, Jones LW. Variations in observable lid wiper epitheliopathy (LWE) staining patterns in wearers of silicone hydrogel lenses. Cont Lens Anterior Eye. 2015;38(6):471-6.
|